This study proves that FrzK is a self-resistant enzyme. Through sequence analysis, homology modeling, crystal structure analysis and point mutation, a series of mutants related to catalytic activity and sensitivity to regulatory inhibitors obtained, indicating that FrzK may pass through Change the conformation of the active site to maintain its PPAT enzyme activity and promote the synthesis of immunosuppressive Qi-)-FR901483
FR901483 an amino acid-derived alkaloid compound isolated from the filamentous fungus Cladobotryum sp. No. 11231. It has immunosuppressive activity. Different from the mechanism of action of other immunosuppressants, it works by inhibiting the synthesis of purine.
In 2019, Professor Tang Yi’s team at the University of California, Los Angeles, identified a biosynthetic gene cluster through heterologous expression and found that the gene frzK encoding phosphoribosyl pyrophosphate amidotransferase (PPAT) may be an autoresistance gene , PPAT enzyme may be the target of 1 (J Am Chem Soc. 2021, 143
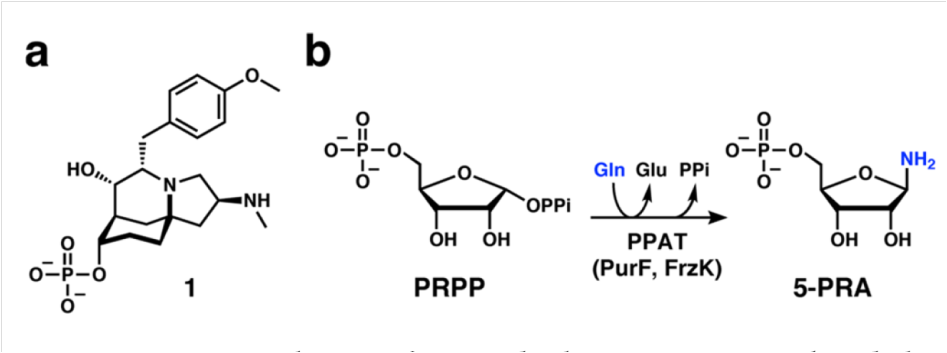
Recently, Professor Kenji Watanabe and Professor Tang Yi of Shizuoka University, Japan, collaborated to clarify the function of FrzK and its homologous protein PurF, and analyzed the mechanism of FrzK in producing resistance.
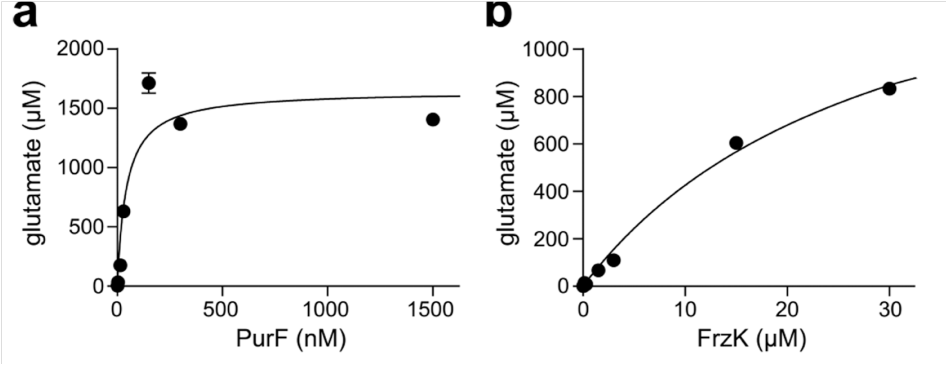
First, FrzK and its homologous protein PurF cloned and expressed. In vitro experiments proved that FrzK and PurF have concentration-dependent activity in catalyzing the formation of 5-phosphoribosyl-1-pyrophosphate (PRPP) into 5-phosphoribosyl-1-amine (5-PRA). , indicating that both have PPAT enzyme activity.
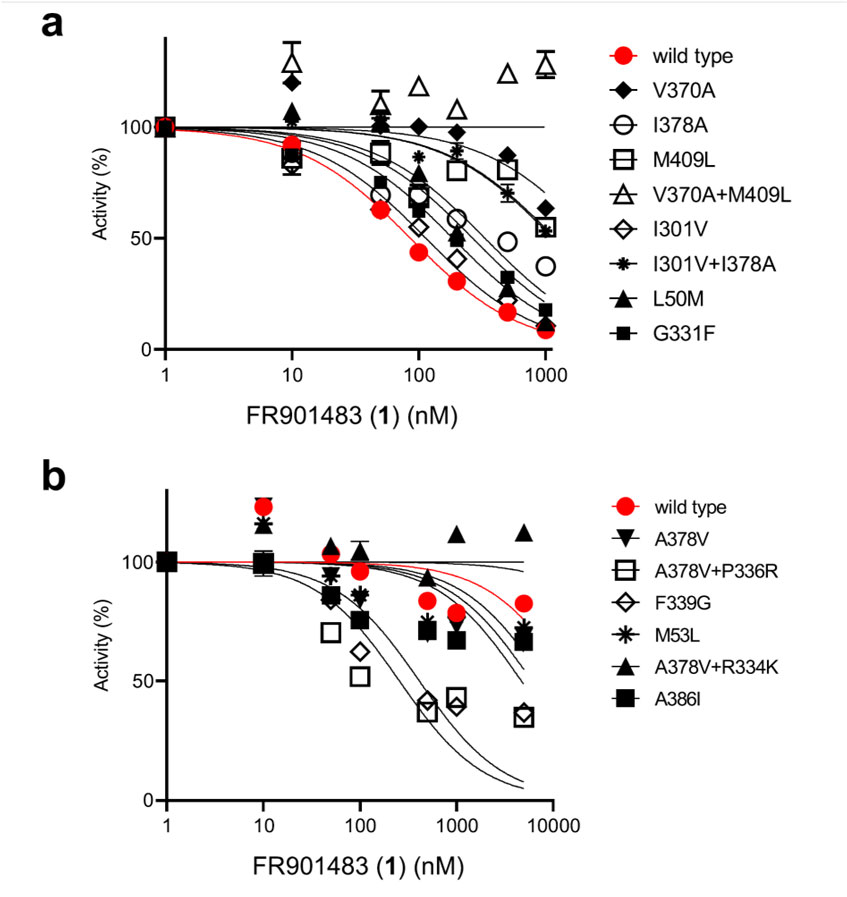
Then different concentrations of fluoride added to the reaction system, and it found that the activity of PurF gradually weakened with the increase of , and basically disappeared when the concentration reached μM; while FrzK able to develop resistance to maintain its activity.
In order to reveal the mechanism of resistance of FrzK, the researchers tried to analyze the crystal structure of FrzK. Because the yield of FrzK was too low, it was difficult to obtain sufficient samples for crystallization, but they obtained a crystal of the complex of PurF and FrzK. After comparison with the known structure, it found that the binding mode of PurF to 1 is very similar to the binding mode of PurF to the feedback inhibitor GMP/AMP. When binding to the substrate, the active site covered by a flexible loop (amino acid residues 328-349). Called the “closed” conformation;
When bound to an inhibitor, the flexible ring is in disorder, exposing the active site to solvent, known as an “open” conformation. At the same time, the C-terminal helix that interacts with the ordered flexible loop is also in a disordered state.
The above results show that the inhibitory mechanism of 1 to PurF is similar to feedback inhibition, except that 1 binds to PurF through the insertion of 4-methoxyphenyl into the phenyl binding pocket. In the active conformation, the active pocket is occupied by the Phe334 phenyl side chain located in the middle of the flexible ring, allowing the flexible ring to protect the active site and substrate from the solvent, inserting the 4-methoxyphenyl group into the phenyl binding The pocket may be one of the key factors that inhibits the production of PurF.
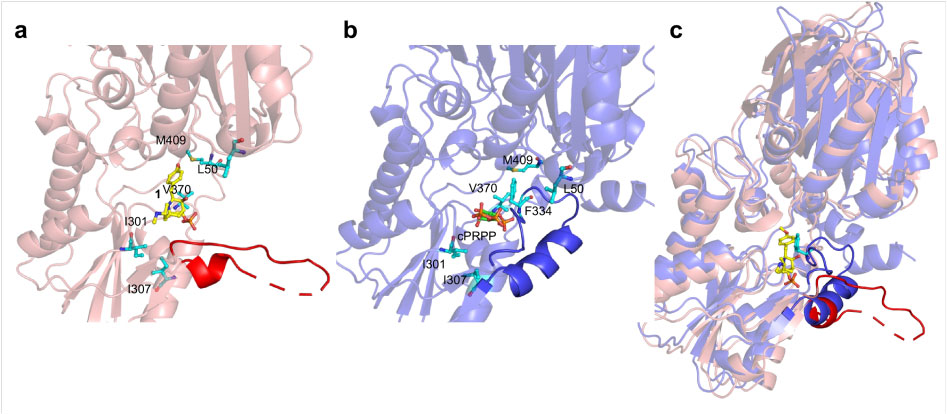
Subsequently, a series of site-specific mutants related to catalytic activity and modulating inhibitor sensitivity were obtained through amino acid sequence analysis, homology modeling, and point mutation analysis.
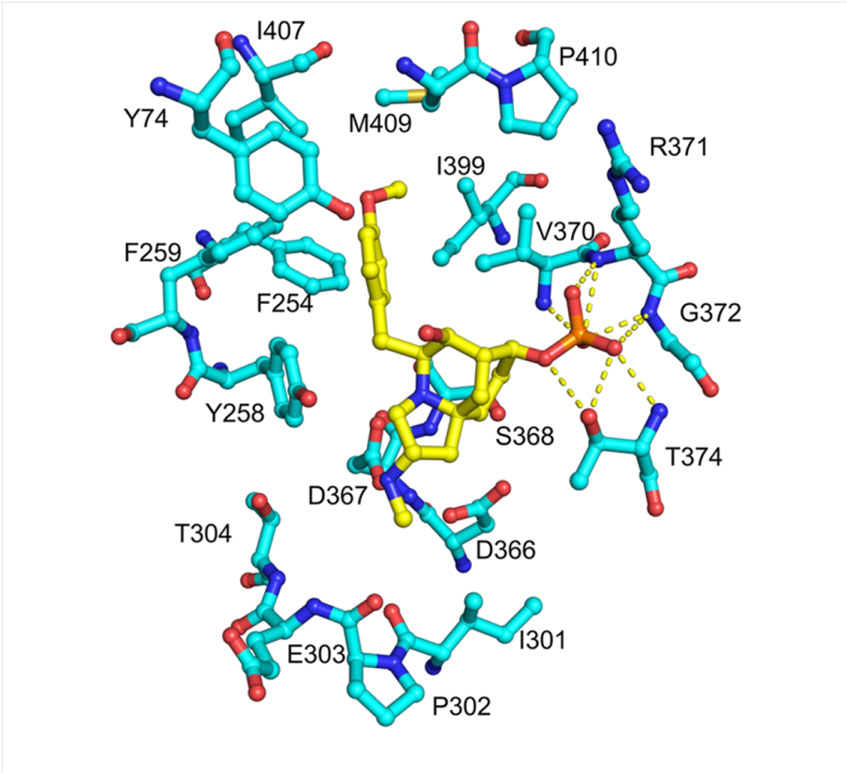
In summary, this study proved through in vitro experiments that frzK is an auto-resistance gene encoding PPAT. Through amino acid sequence analysis, homology modeling, crystal structure analysis and point mutations, a series of genes sensitive to catalytic activity and regulatory inhibitors were obtained. Sex-linked site-specific mutants. The research results indicate that FrzK may restrict its binding to 1 by changing the conformation of the active site, thereby keeping the flexible ring in an active conformation and maintaining its PPAT enzyme activity.