Since the discovery of the double helix structure of DNA, it has been speculated that the gradual destruction of this basic structure is the root cause of aging.
The main basis of this theory has to do with somatic mutations, as somatic mutations accumulate in the genomes of most tissues and species, and this accumulation is associated with multiple features of aging.
In addition to DNA mutations, aging associated with other major types of molecular changes, which has also sparked a debate about which “traits” the root cause of aging.
In particular, recent attention has paid to the strong correlation between age and DNA methylation.
Although there has been different interest in DNA mutation and DNA methylation as aging theories, the relationship between the two is unclear.
On January 13, 2025, Professor Trey Ideker’s team from the Department of Medicine at the University of California, San Diego, published an article in the journal Nature Aging entitled “Somatic mutation as an explanation for epigenetic aging.”
The study found a strong link between the accumulation of sporadic somatic mutations and the widespread changes in methylation observed over the course of life, suggesting that somatic mutations may explain the underlying mechanisms of epigenetic aging.

To investigate the link between somatic mutations and DNA methylation markers, the researchers first analyzed data from human patients included in the TCGA database as well as genome-wide pan-cancer analyses.
The results found that individuals with mutations at the CpG site had significantly less methylation detected compared to individuals without mutations at the same site.
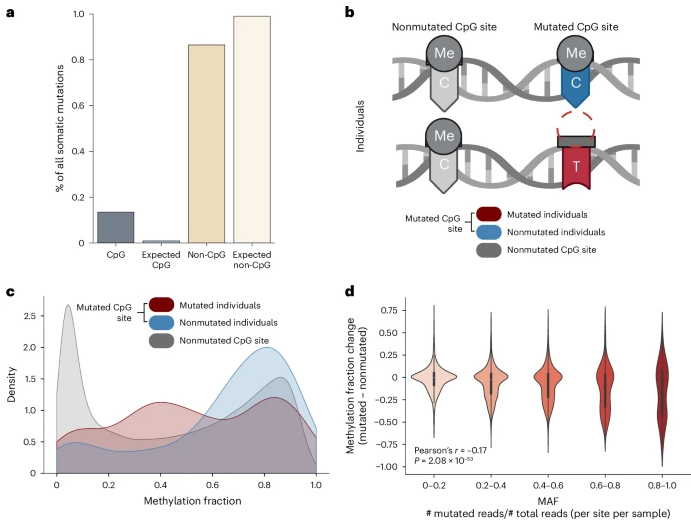
We also found that somatic mutations coincide not only with hypomethylation at the mutant CpG sites, but also with atypical methylation of many CPGS in the surrounding genome.
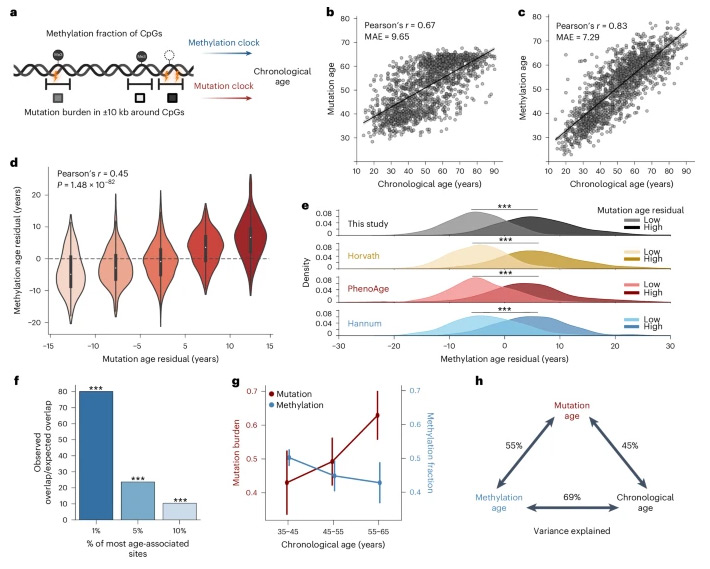
Finally, the researchers found that mutations at any particular CpG site extremely rare in human populations and do not predict age well, but their corresponding CpG methylation scores change regularly, often in ways that age-related.
So the researchers compared two programs that predict a person’s actual age:
The first uses an individual’s CpG methylation value profile, as in previous epigenetic aging models,
The second uses their somatic mutation profiles, including counts of somatic mutations within 10 kb of each identical CpG.
Overall, by evaluating these models using nested cross-validation procedures, somatic mutations found to a good reflection of epigenetic age.
All in all, understanding the causal relationship between somatic mutation, methylation, and aging has important implications for how we can prevent or reverse aging.
In particular, if mutations are the underlying drivers of aging phenotypes, and epigenetic changes simply track this process, then strategies aimed at reversing epigenetic inheritance may simply treat the symptoms, rather than the cause.
References:
https://www.nature.com/articles/s43587-024-00794-x