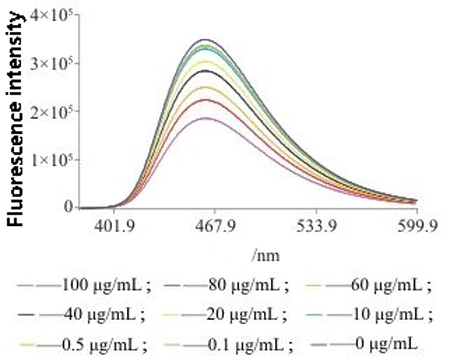
In order to effectively evaluate the biological effects and safety of nanomaterials, since the development of nanomedicine, people have been committed to studying the interface between nanomaterials and various organs of organisms.
Most nanomaterials with large size (>6 nm) and high serum protein binding rate easily captured by the liver and cleared out of the body by the hepatobiliary system.
In contrast, nanomaterials with ultra-small size (< 6 nm) and low serum protein binding rate can be metabolized by the kidney and cleared out of the body with urine, which reduces the accumulation of nanomaterials in the body, and has higher biosafety, so it is concerned.
In recent years, researchers have deeply analyzed the interaction and transport mechanism of renal scavable nanoparticles in the kidney.
But does the liver, the body’s largest detoxification organ, affect and interact with the transport of nanoparticles that can cleared by the kidney? Is still an unknown question.
In the liver, glutathione (GSH) synthesized by hepatocellular parenchymal cells and exported to the perisinusional system of the liver.
Liver glutathione plays an important role in various biotransformations and detoxification of foreign substances.
The team of Du Bujie, a researcher at the Second Affiliated Hospital of South China University of Technology, and Jiang Xingya, a professor at the School of Biomedicine and Science and Engineering of South China University of Technology, conducted a study using nephroscavable IRDye800CW conjugated gold nanoclusters (800CW4-GS18-Au25) as a model. Although 800CW4-GS18-Au25, which can cleared by the kidney, has a very low serum protein binding rate and little accumulation in the liver, its surface ligands gradually altered by hepatic glutathione mediated biotransformation as it passes through the liver.
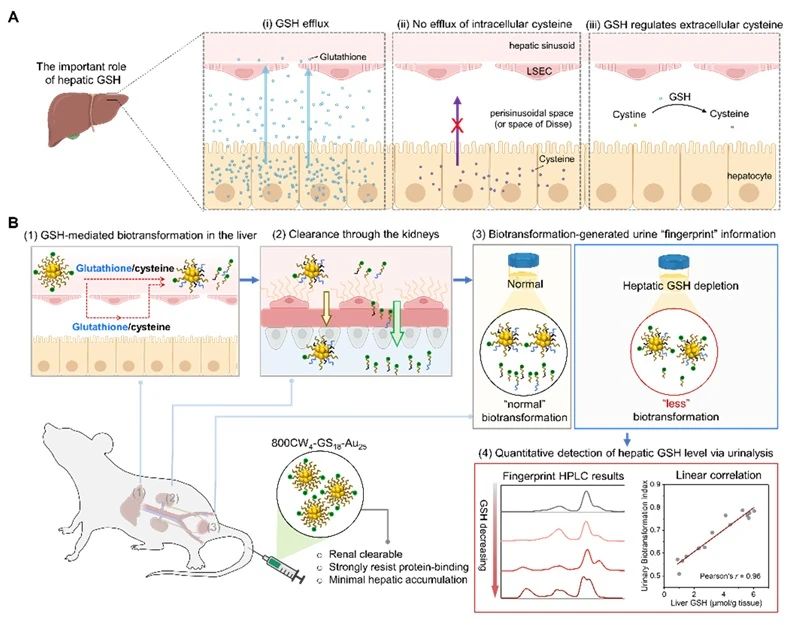
They also observed a linear correlation between the bioconversion index (UBI) in urine and liver glutathione, which allows 800CW4-GS18-Au25 to used as an artificial urine biomarker to quantitatively detect liver glutathione levels.
In this study, we coupled atomic precision gold nanocluster Au25SG18 as the core with the renal-scavenging dye IRDye@800CW to obtain a renal-scavenging nanoprobe 800CW4-GS18-Au25 with near-infrared luminescence, which has good glutathione response ability. The chemical reaction of glutathione with Au-S bond can make corresponding optical changes in the probe, so as to observe the biotransformation and transport behavior of glutathione in vivo.
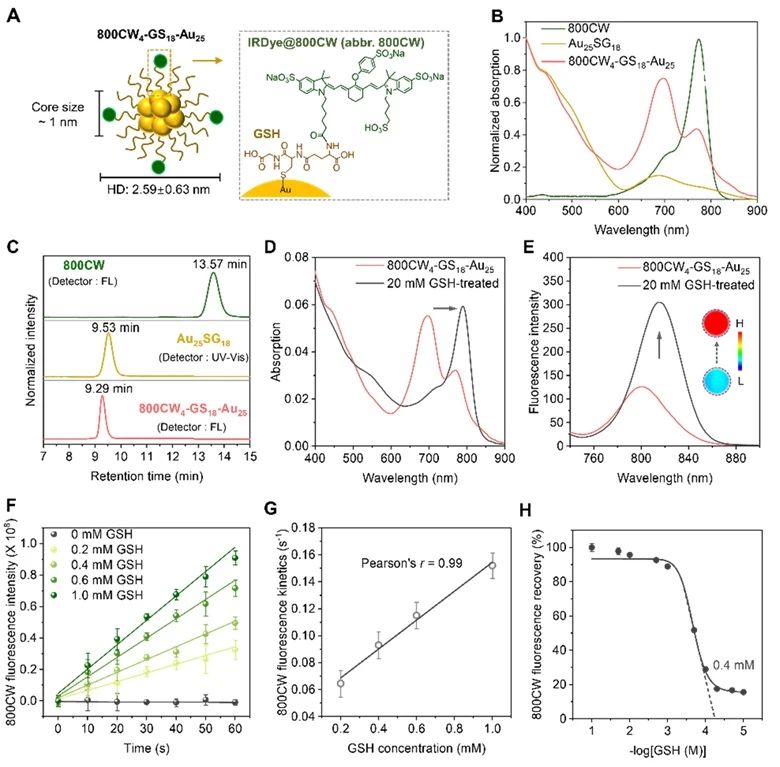
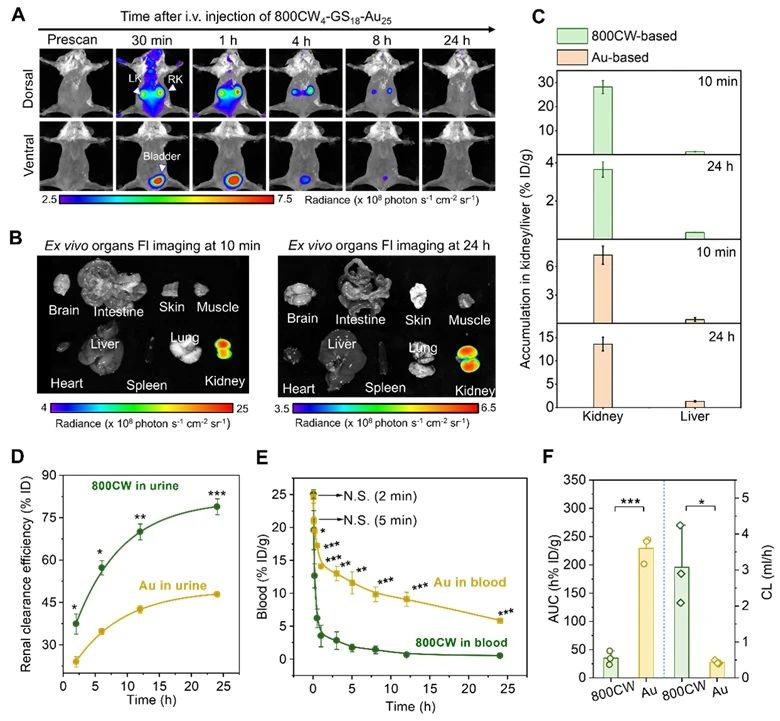
Subsequently, the authors conducted in vivo imaging, biological distribution, renal clearance and pharmacokinetics studies on 800CW4-GS18-Au25 in vivo, and found that the probe had undergone biotransformation before entering the kidney, and the transformation did not occur immediately, but gradually with the administration of the drug.
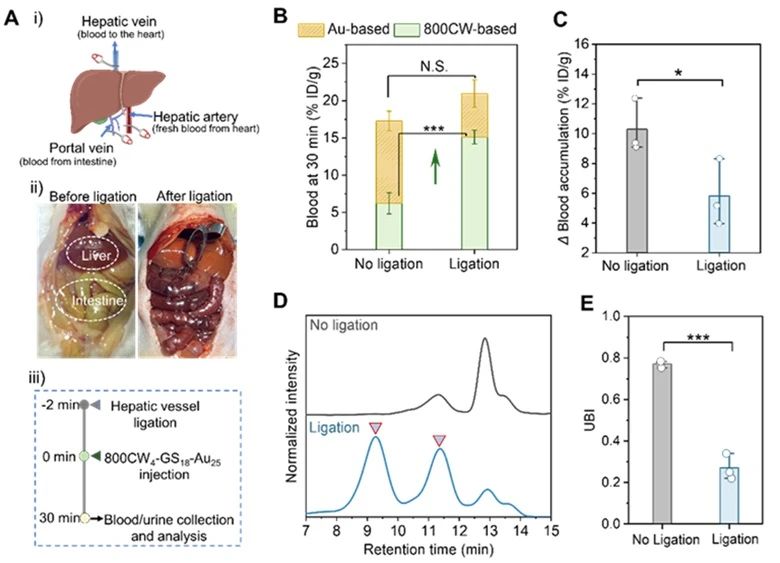
In order to determine the conditions and sites of the probe biotransformation, the authors used diethyl maleate (DEM) to inhibit the production of liver glutathione and its mouse liver vessel ligation model, and explored the important influence of liver glutathione on the biotransformation in renal-clear gold nanoparticles.
It determined that 800CW4-GS18-Au25 biotransformed in the liver by hepatic glutathione.
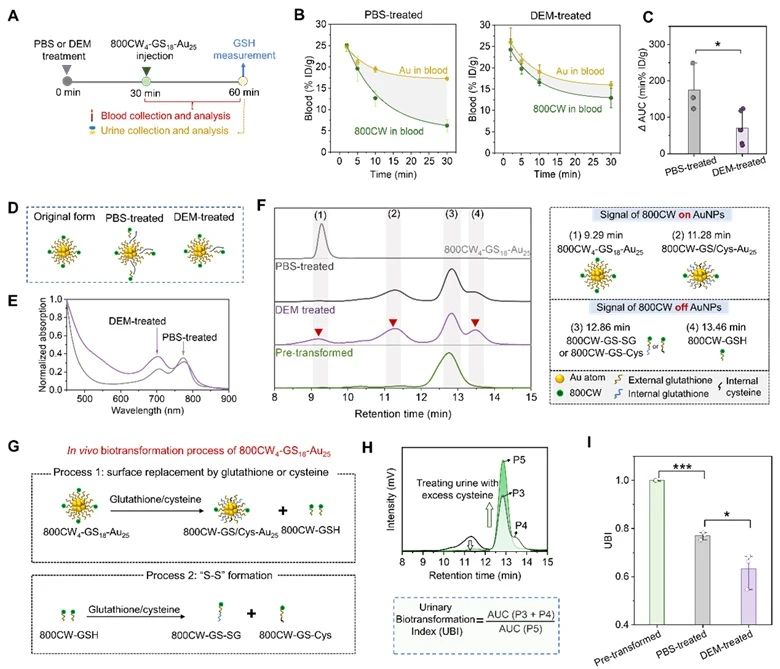
Based on the response of 800CW4-GS18-Au25 to liver glutathione and its renal clearance ability, 800CW4-GS18-AU25 can used as an artificial urine biomarker to achieve non-invasive quantitative assessment of liver glutathione at the living level through urine high performance liquid chromatography, and enable early detection of drug liver injury.
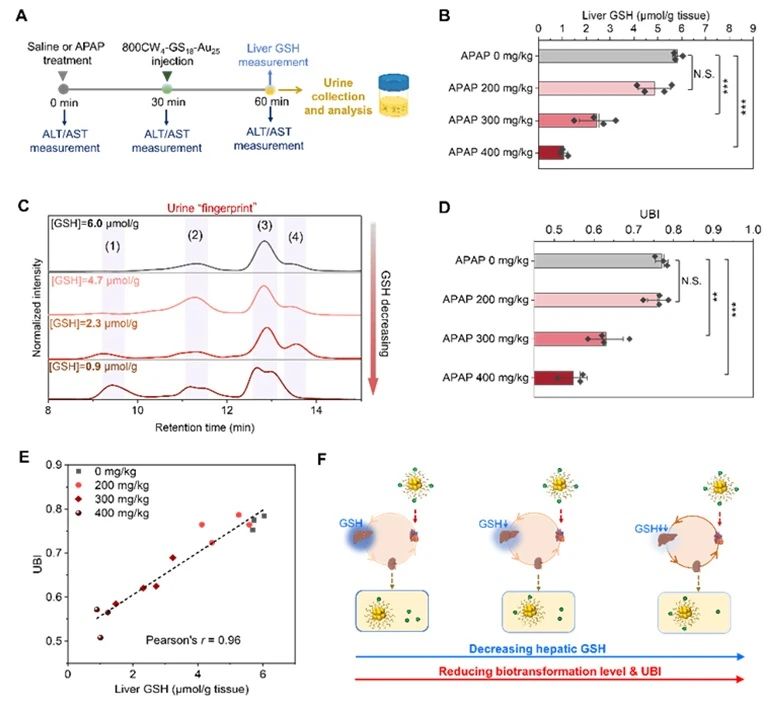
According to the literature, this is the first study to achieve a remote non-invasive quantitative assessment of liver GSH using urine method.
The mechanism of this work has broadened people’s in-depth understanding of the transport of nanoparticles in vivo, and expected to promote the future development of nanoprobes for disease biodetection.