In 2024, an anti-aging method with NAD+ injection is quietly popular in the big health industry, but the scientific principle behind it is less clear.
NAD+ (nicotinamide adenine dinucleotide) is an indispensable molecule in the cell, which is both a helper of energy metabolism and a key role in cell signaling.
It determines whether a cell survives healthily or gradually senescence or even death.
Back in 1906, scientists Arthur Harden and William John Young first discovered NAD while studying yeast and noticed that it speeds up the process of alcohol fermentation.
The main job of NAD+ is to act as a “carrier” of electrons, helping to transport electrons during the cell’s energy production processes (such as glycolysis, tricarboxylic acid cycling, and oxidative phosphorylation) to support cell operation.
NAD+ and NADH are its two states: NAD+ is converted to NADH when it receives electrons, and NADH releases electrons in a cycle.
As people age, the level of NAD+ in the body gradually declines, which can lead to disorders in cell metabolism and may increase the risk of chronic diseases.
The study found that boosting NAD+ levels in experimental organisms not only delayed aging, but also improved health and even extended life.
As a result, scientists are actively exploring ways to boost NAD+ levels in either endogenous or exogenous ways.
Next, this article will highlight the importance of NAD+, the clinical evidence from relevant studies, and specific strategies for enhancing NAD+.

introduction
The study of NAD+ is of great interest because scientists have found that it is essential for many functions of living organisms.
In particular, supplementing NAD+ with its precursors (i.e. its “raw material”) is increasingly becoming a popular form of health intervention.
There is growing evidence that NAD+ levels decline with age, and this change is strongly associated with age-related frailty and multiple diseases.
NAD+ plays multiple roles in the mitochondria, the cell’s energy factory. It is a “helper” involved in energy production, such as supporting the work of enzymes in the electron transport chain, helping the cell to produce ATP (the energy currency of the cell).
It is also a key component in the tricarboxylic acid cycle, providing essential support for important enzymes.
In addition, NAD+ is involved in REDOX reactions to maintain energy balance within cells.
It is an essential molecular building block for specific proteins such as Sirtuins, PARPs, and CD38.
With age, the activity of these proteins increases significantly, and the consumption of NAD+ increases accordingly.
Because of the broad role of NAD+, scientists believe that there is great potential to treat many health problems by supplementing the precursors of NAD+.
It is not yet clear which precursor is the best choice, as well as the specific dosage of supplementation.
Many factors affect precursor metabolism, such as an individual’s digestive system and gut flora, making it difficult to develop standard recommendations for different populations.
The recommended daily intake of niacin is 16 mg for men and 14 mg for women, but 60 mg of tryptophan (one of the essential amino acids) is required to produce 1 mg of niacin.
The study found that by supplementing the intermediate metabolites of NAD+, rather than relying solely on diet, NAD+ levels can be significantly increased at higher doses, with multiple health benefits and possibly even therapeutic effects.
Synthesis of NAD+
NAD+ is synthesized in animals through three main pathways, each using a different molecular raw material to complete the synthesis process.
1. De novo synthesis
De novo means “from scratch”, i.e. the process of synthesising NAD+ from simple raw materials such as tryptophan:
- The starting point is tryptophan, which is converted into kynuridine by an enzyme (tryptophan 2, 3-oxygenase) and enters the cell via the cell transporter (SLC6A19).
- Kynurenine is further converted into an intermediate (ACMS), which later forms quinolinic acid (QA).
- Quinolinic acid is converted to nicotinic acid mononucleotide (NaMN) in a series of reactions, and then converted to nicotinic acid adenine dinucleotide (NaAD) by NMNAT enzyme.
- Finally, NaAD is converted to NAD+ by NAD+ synthase (NADSYN) using ATP.
Each NMNAT enzyme is distributed differently within the cell:
- NMNAT1 is located in the nucleus,
- NMNAT2 is in the cytoplasm of neurons,
- NMNAT3 is found in mitochondria.
2. Preiss-Handler path
This pathway uses niacin (NA) as a feedstock and operates directly in the cytoplasm:
- Niacin enters the cell via specific transporters such as SLC5A8 and SLC22A3.
- It is then converted to NaMN by niacin phosphoribosyl transferase (NAPRT), a key step in the entire pathway.
- Next, NaMN follows the next steps of the De novo path, first converting to NaAD and finally to NAD+.
The path takes its name from the two scientists who first described the process in 1958: David Preiss and Joseph Handler.
3. Salvage path
Salvage means “to recover” and refers to the synthesis of NAD+ by recycling molecules already in the cell, such as NMN and NAM:
- Nicotinamide ribose (NR) enters the cell via transporters such as ENT1 and ENT2, while nicotinamide (NAM) diffuses directly into the cell.
- NAM is converted to NMN by an enzyme (NAMPT), while NR is converted to NMN by the NRK enzyme.
- The resulting NMN is again converted to NAD+ by the enzyme NMNAT.
In addition, the NRH Salvage path is a newly discovered branch:
Dihydronicotinamide ribose (NRH) enters the cell through the cell membrane, is phosphorylated to NMNH by a kinase (possibly adenosine kinase), and finally converted to NADH by NMNAT and further oxidized to NAD+.
These three synthesis methods together ensure the supply of NAD+ in the cell, where De novo synthesis starts with tryptophan, the Preiss-Handler path utilizes niacin, and the Salvage path maximizes the recovery of existing molecules in the cell.
The flexibility of these pathways allows NAD+ to maintain the normal functioning of cellular energy metabolism and repair functions.
Function of NAD+ : Key role in cellular activity
NAD+ is the “fuel” for many important protein families, helping them to accomplish specific tasks.
Here are some of the main NAD+ dependent features and what they do:
1. DNA’s “repairman” : PARPs
PARPs are enzymes that repair DNA damage.
When cells experience DNA damage, PARPs split NAD+ to produce ADP-ribose and niacinamide (NAM).
These molecules attach to histones and other proteins and start the DNA repair process:
PARP1: Mainly responsible for DNA repair, accounting for 90% of all PARP activity, especially in genotoxic environments.
PARP2: Recognizes breaks in DNA strands and convenes repair complexes.
Both consume NAD+ and release NAM during the repair process.
2. guardians of aging and longevity: Sirtuins
Sirtuins are a class of NAD+ dependent enzymes that are responsible for removing chemical markers (such as acetyl groups) from proteins and have a profound impact on cell metabolism and lifespan:
There are seven Sirtuins proteins, among which SIRT1, SIRT6 and SIRT7 are located in the nucleus and participate in DNA repair and gene regulation. For example:
- SIRT1 activates repair proteins that promote the repair of DNA double-strand breaks.
- SIRT6 enhances repair efficiency in collaboration with PARP1.
- SIRT7 helps the site of DNA damage recruit other repair factors.
These enzymes also help cells maintain energy balance by regulating mitochondrial production and autophagy, which is particularly important in tissues with high energy requirements, such as the brain and heart.
Loss of Sirtuins function associated with genomic instability and shortened lifespan.
3. “Guardians” of mitochondrial metabolism: SIRT3, SIRT4 and SIRT5
SIRT3: Helps brain neurons adapt to metabolic and exercise stress and is essential for energy management.
SIRT4 and SIRT5: participate in the regulation of mitochondrial metabolism to ensure the balance of cell energy supply.
Dysregulation of Sirtuins may associated with cancer.
For example, SIRT3 can induce apoptosis by regulating mutant tumor suppressors, and overexpression of SIRT1 and SIRT7 may associated with the development of cancer.
4. “switch” that regulates calcium signaling: CD38
CD38 is a transmembrane protein that is both a receptor and an enzyme. It produces cyclic adenosine diphosphate ribose (cADPR) by breaking down NAD+, an important signaling molecule that regulates calcium levels within cells:
CD38 not only found on the cell surface, but also in internal structures such as the nucleus, highlighting its versatility.
With age, CD38 activity increases, leading to a decline in NAD+ levels and affecting cell function.
In mice, blocking CD38 restored NAD+ levels and improved mitochondrial health.
NAD+ Precursors: “raw material” of the body’s energy cycle
Here are a few common NAD+ precursors and what they do:
1. Niacin (NA)
Niacin is a basic component of NAD+, which promotes energy production in the body.
Scientist Conrad Elvehjem’s discovery in the early 1900s that niacin could treat pellagra (a disease that causes skin inflammation, diarrhea, and dementia) in dogs spurred the development of dietary supplements.
In 1955, it found that taking 3 grams of niacin per day could lower cholesterol.
Niacin can also improve problems such as type 2 diabetes, obesity and atherosclerosis. In some disease models, niacin was able to significantly increase NAD+ levels and improve mitochondrial function.
High doses of niacin may trigger a flushing reaction, but appropriate doses are usually safe.
2. Niacinamide (NAM)
Nicotinamide is a form of vitamin B3 that produces NAD+ through a recycling mechanism.
NAM has found to help increase NAD+ levels in the brain and reduce brain damage after stroke.
The conversion rate of NAM limited, and high doses may inhibit the activity of other important enzymes and even lead to toxicity risks.
3. Nicotinamide mononucleotide (NMN)
NMN is a nucleotide that is directly converted to NAD+.
NMN naturally found in foods such as avocado and broccoli, and can quickly absorbed and distributed to various tissues after entering the body.
Studies have shown that NMN supplementation improves mitochondrial health and enhances cell function, especially in diseases related to aging and energy metabolism.
When NAD+ levels drop, the transport mechanism of NMN “activated” to help the body quickly restore energy balance.
4. Nicotinamide ribose (NR)
NR is another highly potent precursor of NAD+. Similar to NMN, NR can enter cells without complex transformation and rapidly increase NAD+ levels.
NR has shown to enhance mitochondrial function, increase stem cell activity, and help slow weight gain caused by high-fat diets in dietary studies.
Less NR found in natural foods (such as milk) and more obtained through supplements.
5. Nicotinamide ribose (NRH)
NRH is a newly discovered precursor of NAD+ that is more efficient than NMN and NR.
NRH can increase the level of NAD+ in cells several times, significantly improving metabolism.
It uses a different synthetic pathway than other precursors, opening up new directions for NAD+ complementary research.
NAD+ and premature aging and neurological diseases
senilism
NAD+ depletion plays an important role in both normal aging and accelerated age-related diseases.
When NAD+ levels drop or NAD+ dependent enzymes altered, each of the original aging features affected.
With age, NAD+ levels decline, and oxidative stress generated by mitochondrial electron transfer involving NADPH activity leads to the accumulation of abnormal protein aggregates.
Decreased levels of NAD+ lead to decreased activity of sirtuins, particularly SIRT1, SIRT3, and SIRT6, which produce high concentrations of niacinamide (NAM) byproducts that inhibit the ability of these proteins to assist DNA repair.
Decreased NAD+ levels also adversely affect cellular energy metabolism, particularly in maintenance, repair, and mitochondrial biogenesis.
Nucleo-mitochondrial communication (regulated by PGC-1α) also negatively affected by the decreased availability of NAD+.
In old wild-type mice, reduced levels of NAD+ triggered age-related declines in mitochondrial bioproduction due to impaired SIRT1-PGC-1α signaling.
Due to the damage of mitochondrial autophagy mechanism, the number of defective mitochondria increases, producing a large number of reactive oxygen species (ROS), causing damage to the organelles, and thus leading to accelerated aging.
Neurodegenerative disease
Neurodegenerative diseases characterized by the gradual degeneration of specific populations of neurons over time.
Diseases that cause neurodegeneration can be classified according to major clinical features (such as motor nerve disease or dementia), molecular properties, or physiological patterns of neurodegeneration.
The most common type of dementia Alzheimer’s disease (AD), which affects one in nine people over the age of 65 (10.8%) and expected to grow from $345 billion in care costs today to $1 trillion by 2050.
Alzheimer’s disease characterized by memory loss, aphasia, disorientation, and a variety of other neurocognitive dysfunction.
Pathological features of AD include extracellular plaques of amyloid beta protein (Aβ) and intracellular neurofibrillary tangles composed of hyperphosphorylated Tau protein.
In preclinical studies, NAD+ supplementation has shown to reduce the pathological features and DNA damage response of AD.
In a DNA-repair deficient AD model, NR supplementation reduced Tau pathology, neuroinflammation, and improved synaptic transmission and cognitive function.
NR has also shown to be effective in another mouse model of AD (APP/PS1), alleviating neuroinflammation and cell aging.
In another study, a 250 mg/kg daily application of NR alleviated amyloid beta toxicity in the cortex and hippocampus of a Tg2576 AD mouse model through PGC-1α-mediated BACE1 degradation.
The brain neurons of AD patients have accumulated dysfunctional or damaged mitochondria due to the absence of mitochondrial autophagy.
By supplementing NAD+ preforms, the balance between mitochondrial autophagy and mitochondrial biogenesis can improve the pathological features of AD via either the NAD+-SIRT1-PGC-1α pathway or the DAF-16/FOXO pathway.
Taken together, these results suggest that NAD+ supplementation is a safe and effective anti-AD strategy.
Parkinson’s disease (PD) is a neurodegenerative disease that progressively worsens with age, causing patients to have severe difficulties in controlling their body movements.
The exact cause of PD not fully understood, but research suggests that it may caused by a combination of genetic factors (such as SNCA gene mutations) and environmental factors (such as traumatic brain injury).
Mitochondrial protein and lysosomal enzyme β-glucoceresidase (GBA) gene mutations and changes in NAD+ metabolism have also suggested as causes of PD.
In a Drosophila PD model carrying GBA mutations, the application of NR was able to prevent dopaminergic neuron loss and improve movement disorders.
Dyskinesia results from the loss of dopaminergic neurons in the substantia nigra, with abnormal accumulation of alpha-synuclein fibers in the cell bodies and neuronal processes of these neurons.
Cells from PD patients exhibit overactivation of SARM1, whose TIR domain induces axon degeneration and depletion of axon NAD+, while α-synuclein competes with NAD+ to bind to the glycosyl-3-phosphate dehydrogenase, co-localizing with α-synuclein in the Lewis body.
Given the observed decrease in NAD+ levels in multiple models of PD and the initial success of precursor supplementation in PD models, the researchers hope that supplementation with NAD+ will serve as a potential target for treating this disease.
There are two ongoing clinical trials using a proprietary formulation of the crystalline form of β-nicotinamide mononucleotide MIB-626 to treat patients with AD.
The first trial will evaluate whether MIB-626 can cross the blood-brain barrier and whether 90 days of oral treatment can boost NAD+ levels in the brain.
Next, a 180-day trial will conducted to assess whether biomarkers of amyloid deposition, neuron/axon degradation, synaptic function, and neuroinflammation improve (NCT05040321).
A separate trial (NCT04430517) conducted to investigate the effects of NR on brain energy metabolism, oxidative stress, and cognitive function in patients with mild AD and cognitive impairment.
With regard to PD, there currently two randomized, double-blind clinical trials underway to evaluate the efficacy of NAD+ supplementation in patients with PD.
The trials named NAD-PARK (NCT03816020) and NO-PARK (NCT03568968).
The NAD-Park trial had patients take NR supplements orally for 4 weeks, and preliminary data indicated increased levels of NAD+ in the brain and altered brain metabolism in PD patients.
Further trial results may support the use of NAD+ supplementation as a clinical intervention to treat these debilitating conditions.
How to improve NAD+
With the deepening of research, people have a more comprehensive understanding of the role of NAD+ in maintaining cell function and delaying aging, so the development and application of strategies to improve the level of NAD+ has become a research hotspot.
These methods mainly include precursor supplementation, enzyme activity regulation and lifestyle intervention.
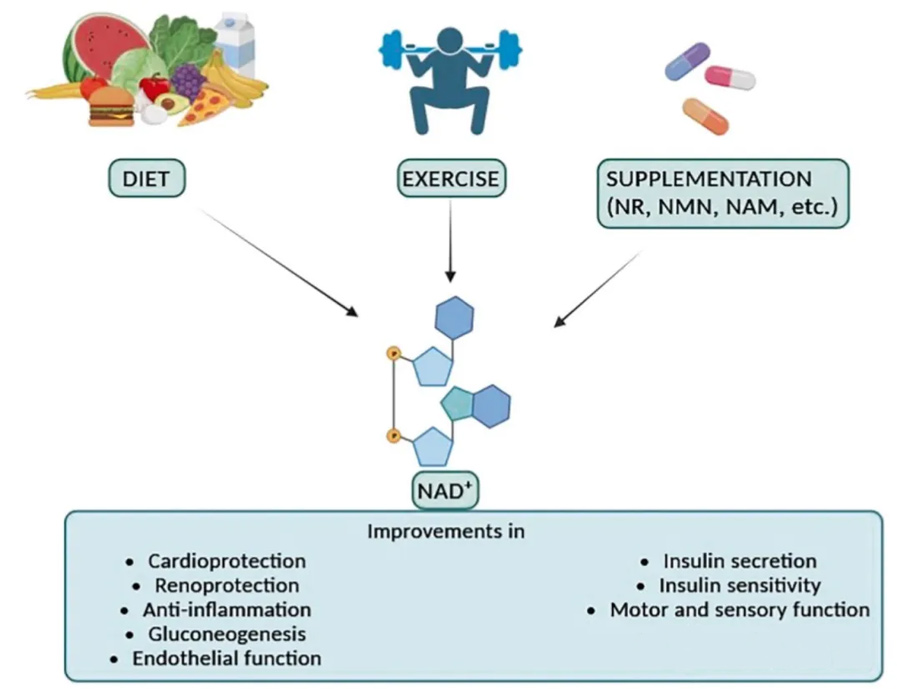
Improvement of human physiological function after increasing NAD+ level through diet, exercise and NAD+ precursor supplements (such as NR, NMN, NAM)
NAD+ enhancement benefits multiple physiological functions and health, and the combination of diet, exercise, and supplement strategies can significantly improve fitness and survival.
Precursor supplementation
Precursor supplementation is the most direct way to improve the level of NAD+, especially in the Salvage path, where these precursors generate NAD+ through multi-step metabolism.
Common NAD+ precursors include nicotinamide mononucleotides (NMN), nicotinamide ribose (NR), niacin (NA), and nicotinamide (NAM).
1. Nicotinamide mononucleotide (NMN)
NMN is a direct precursor of NAD+ biosynthesis and can be converted to NAD+ through the Salvage path.
Studies have shown that oral NMN rapidly absorbed by the intestine and enters cells via the SLC12A8 transporter.
In mouse models, NMN supplementation significantly increased NAD+ levels in a variety of tissues, such as muscle, liver, and brain, and improved age-related conditions, including reduced insulin sensitivity, mitochondrial dysfunction, and inflammation.
2. Nicotinamide ribose (NR)
NR is a water-soluble vitamin B3, which can be rapidly converted to NMN by NRK kinase, and then further to NAD+.
NR has shown promising results in improving mitochondrial function, protecting neurons and enhancing stem cell regeneration.
In preclinical studies, NR was able to alleviate pathological features in mouse models of Alzheimer’s disease (AD) and Parkinson’s disease (PD) and improve cognitive and motor function.
3. Niacin (NA)
Niacin is a traditional form of vitamin B3 that generates NAD+ via the Preiss-Handler path.
NA can not only increase the level of NAD+, but also show the effect of improving lipid metabolism and lowering cholesterol.
A significant side effect of NA is causing a flushing reaction, which may limit its use in high-dose supplementation.
4. Niacinamide (NAM)
NAM is the main metabolite of NAD+ hydrolysis and is recycled to produce NAD+ through NAMPT catalysis.
NAM has used to improve cerebral vascular disease and recovery after stroke.
High doses of NAM may inhibit the activity of PARP and sirtuins, limiting their application potential.
Regulation of enzyme activity
In addition to directly supplementing NAD+ in vitro, regulating enzyme activity related to NAD+ metabolism is also an effective strategy, especially targeting those depleting enzymes that lead to NAD+ depletion.
1. CD38 inhibition
CD38 is a NADase whose activity increases with age and significantly reduces intracellular NAD+ levels.
but also negatively affects the metabolism of NMN and NR.
CD38 not only consumes NAD+ directly,
Studies of CD38 knockout mice showed significant improvements in both NAD+ levels and mitochondrial function.
CD38 inhibitors, such as approamide molecules, have shown to significantly increase NAD+ levels and improve mitochondrial function.
2. PARP inhibition
PARP consumes a large amount of NAD+ in DNA damage repair.
With age, PARP activity increases, further accelerating the depletion of NAD+.
PARP inhibitors are able to protect NAD+ levels by reducing its consumption, while enhancing the cells’ DNA repair capabilities.
These inhibitors have shown potential in research related to anti-cancer and anti-aging.
3. NAMPT activation
NAMPT is the rate-limiting enzyme in the Salvage pathway, and its activity directly determines the efficiency of converting NAM into NAD+.
By regulating the activity of NAMPT, the biosynthesis rate of NAD+ can significantly increased.
This approach is emerging as a new direction for the development of NAD+ enhancers.
Lifestyle intervention
One way to naturally boost NAD+ levels is to make lifestyle changes.
Studies have shown that specific diet, exercise, and other interventions can effectively increase NAD+ levels and improve mitochondrial function.
1. Caloric restriction
Caloric restriction helps to maintain or increase NAD+ levels by reducing NAD+ consumption and activating the sirtuins pathway.
This intervention not only extended the lifespan of mice, but also improved metabolic health in humans.
2. movement
Regular exercise can significantly increase NAD+ levels in skeletal muscle.
This enhancement mainly achieved by increasing the expression of genes associated with mitochondrial biogenesis, such as PGC-1α and NRF1, thereby improving mitochondrial function and overall health.
3. Dietary regulation
Foods rich in NAD+ precursors, such as NMN and NR, including avocado, broccoli, and milk, may be beneficial for a natural boost in NAD+ levels.
Polyphenols, such as resveratrol, have shown to indirectly raise NAD+ levels by activating sirtuins.
Some problems in NAD+ application
While the strategy to raise the level of NAD+ shows great potential, there still many issues that need to addressed.
Here are some key challenges and directions for future research:
1. Dosage and safety issues
Although NAD+ precursor supplements, such as NMN and NR, have shown to well tolerated in many studies, the safety of long-term use still not fully understood:
- High dose risk: High doses of niacinamide (NAM) or niacin (NA) may inhibit the activity of important enzymes such as PARP and SIRT. These enzymes are essential for DNA repair and maintenance of cell metabolism, and their inhibition may negatively affect cell function.
- Side effects: For example, high doses of NA may cause flushing reactions, which may limit its widespread clinical use.
- Research needs: More in-depth studies needed in the future to determine the optimal dose range for each precursor and to systematically evaluate the potential side effects of long-term use to ensure its safety.
2. Disease-specific problem
The role of NAD+ augmentation has been demonstrated in certain diseases, such as:
- Clear benefits: In neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, as well as metabolic disorders, NAD+ enhancement has shown potential to improve cognitive function and metabolic health.
- Areas of uncertainty: In other diseases, such as cancer, the effects of elevated NAD+ levels are unknown. In particular, NAD+ may enhance the metabolic activity of tumor cells, thereby promoting their growth.
- Research needs: It is necessary to further study the specific role of NAD+ in different disease backgrounds, clarify its scope of application in treatment, and avoid potential risks.
3. Individual difference problem
Each person’s response to NAD+ enhancement strategies may vary significantly depending on their genetic and metabolic status:
- Absorption and metabolic efficiency: Some people may have low absorption of NMN or NR, which can affect treatment effectiveness.
- Individual needs: Factors such as genetic background, age, and health status may affect the efficacy of NAD+ supplementation.
- Solutions: In the future, personalized NAD+ enhancement regiments will need to developed, with dosages and uses tailored to the specific needs of patients to achieve optimal efficacy.
NAD+ is a key molecule in maintaining cell metabolism, gene stability and healthy aging, and its decreased levels are closely associated with a variety of age-related diseases.
Enhancing NAD+ levels through precursor supplementation, enzyme activity regulation and lifestyle intervention provide important possibilities for delaying aging and treating chronic diseases.
However, issues such as dose, safety, disease-specific and individual differences still need to overcome to play the role of NAD+, so that it can widely used.
When applying NAD+, Xiaobian recommends focusing on the following aspects:
- Optimize the formulation and dosage of NAD+ precursors to increase efficacy and minimize side effects.
- Develop novel NAD+ enhancing drugs that target the regulation of key enzymes involved in NAD+ metabolism.
- Individualized treatment, with customized NAD+ enhancement strategies based on the patient’s genetic background and metabolic status.
- Long-term clinical trials to evaluate the safety, efficacy and specific effects of NAD+ enhancement strategies in different disease Settings.




