Mycobacterium tuberculosis (Mtb) causes chronic lung infections through the air, and the problem of drug resistance makes treatment more difficult.
Tuberculosis (TB) is one of the world’s most deadly infectious diseases, killing more than a million people each year.
The BCG vaccine, the only approved TB vaccine, is effective against disseminated TB in children but provides limited protection (only 50%) against TB in adults.
BCG, the only approved TB vaccine, prevents severe TB in children through attenuated Mycobacterium bovis, but its protection rate against adult TB is less than 50%, which has remained unsolved for 100 years.
Recently, research teams from Cornell University, Harvard University, and the University of Pittsburgh have carried out a number of innovative studies to work on the design of genetically engineered strains of tuberculosis.
In two concurrent papers published in Nature Microbiology, researchers modified mycobacteria to have built-in kill switches to improve the safety of tuberculosis vaccines.
The first article was titled “A BCG kill switch strain protects against Mycobacterium tuberculosis in mice and non-human primates with. improved safety and immunogenicity, “led by Professors Dirk Schnappinger and Sabine Ehrt of Weill Cornell Medical College and JoAnne Flynn of the University of Pittsburgh.
Developed a safer engineered BCG that strongly prevents Mtb infection;
The second article is titled Engineered Mycobacterium tuberculosis triple-kill-switch strain provides controlled tuberculosis infection in animal models, led by Sabine Ehrt and Dirk Schnappinger, along with Professor Sarah Fortune and Professor Eric Rubin of the Harvard School of Public Health, obtained a promising engineered strain of Mycobacterium tuberculosis.
It can be used as a candidate strain for human attack research.


The first study focused on making the high-dose intravenous BCG vaccine safer while maintaining its ability to stimulate immunity.
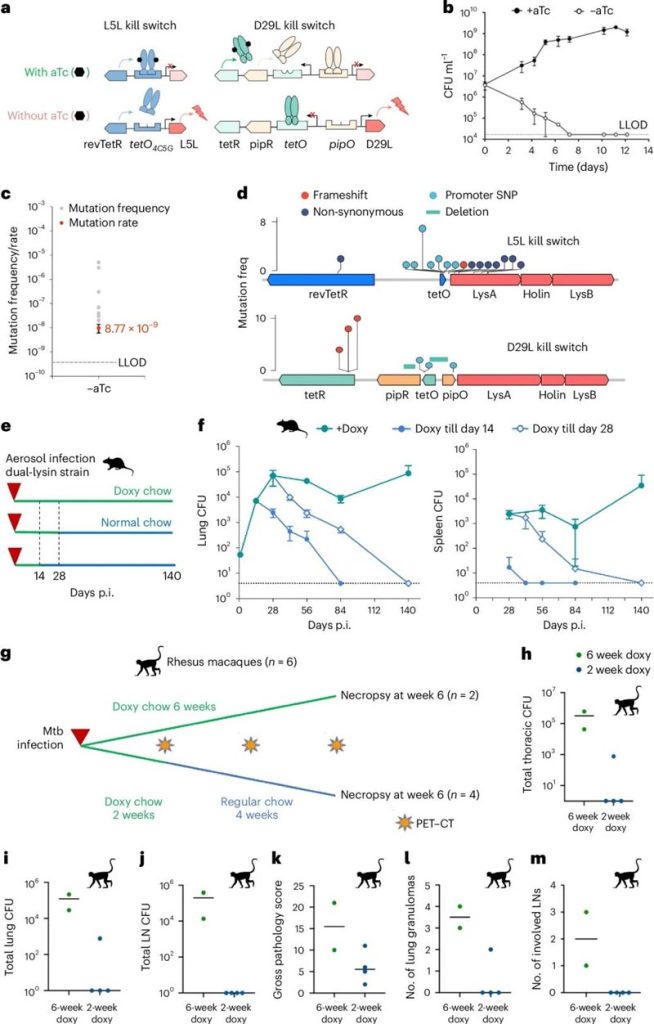
Based on the BCG vaccine, the researchers set up a built-in safety mechanism – the BCG vaccine “kill switch” to avoid the possibility of accidental self-infection with attenuated mycobacteria.
The researchers designed two built-in mechanisms to obtain two genetically engineered strains:
BCG-TetOFF-DL, which requires tetracycline (aTc) or doxycycline (doxy) to grow, and BCG-TetON-DL, which killed by these antibiotics.
To build the strain, the researchers utilized lysin operons found in the genome of mycobacteriophages, which encode lysinases capable of killing the bacteria’s host cells.
Induced cell lysis controlled by engineered expression of two lysins in the BCG strain, resulting in BCG killed in vitro while the frequency of escaping mutants reduced.
Experiments in mice have shown that BCG vaccines containing this dual safety switch protect animals against tuberculosis with comparable efficacy to standard BCG vaccines, but with the advantage of faster elimination and greater safety, even for immunocompromised mice.
“The modified BCG elicits a similar immune response to the wild-type BCG and provides similar protection to the mice against attack by Mycobacterium tuberculosis,” the researchers wrote.
In rhesus monkeys, the upgraded self-destruct BCG vaccine elicits a stronger immune response and provides better protection against tuberculosis than standard intravenous BCG.
After 8 weeks of infection with live mycobacterium tuberculosis, there no detectable lung inflammation in rhesus monkeys vaccinated with the upgraded BCG vaccine.
In addition, six of the eight rhesus monkeys found no recoverable traces of live mycobacterium tuberculosis, compared with two of the eight rhesus monkeys given standard BCG intravenously.
“Our data suggest that this’ kill switch ‘BCG strain induces a stronger CD4+ T cell response in the lung and may be more protective in rhesus monkeys than WT BCG.”
Six of the eight rhesus monkeys developed a bactericidal immunity, compared with only two of the eight rhesus monkeys vaccinated with wild-type BCG.
The ‘kill switch’ BCG strain may provide additional safety and strong protection against mycobacterium tuberculosis infection.”

In another study, the researchers engineered a strain of Mycobacterium tuberculosis with three “kill switches” :
Two phage lysin operons negatively regulated by aTc or doxycycline and a degradation domain -NadE fusion.
This triple kill switch (TKS) strain of Mycobacterium tuberculosis grows in the presence of aTc and TMP, but does not grow in the absence of aTc and TMP.
In vivo, M. tuberculosis TKS produces infections comparable to the wild type in mice receiving antibiotics aTc and TMP, and rapidly eradicated in the absence of aTc and TMP, with no recurrence observed.
TKS Mtb exhibited a low escape rate (less than 10-10 per genome per generation) and rapid elimination kinetics, both of which key safety factors for Mtb strains used in human challenge studies of TB.
Although these results are excellent in animal models, a great deal of follow-up research needed to successfully translate this technique into clinical applications.
Human challenge testing is an essential step for scientists to confirm the safety and effectiveness of this genetically engineered strain in humans.
While the available animal data is very promising, it is only in controlled clinical trials that the performance of this vaccine in humans can truly evaluated.
The research team plans to use the current technology platform to further expand human trials over the next few years, ensuring that the strictest safety standards met at every step.
References:
- 1.Alexander A. Smith et al, A BCG kill switch strain protects against Mycobacterium tuberculosis in mice and non-human primates with improved safety and immunogenicity, Nature Microbiology (2025). DOI: 10.1038/s41564-024-01895-4
- 2.Xin Wang et al, Engineered Mycobacterium tuberculosis triple-kill-switch strain provides controlled tuberculosis infection in animal models, Nature Microbiology (2025). DOI: 10.1038/s41564-024-01913-5