Adenosinetriphosphate (ATP), first discovered in 1929, is the common energy currency of all living cells.
The field of ATP signaling has made many advances over the past century.
ATP, as an ideal extracellular messenger, has the following four characteristics:
- ① High signal-to-noise ratio: extracellular concentration is close to zero;
- (2) High hydrophilicity, can be diffused without restriction in the water interstitial environment;
- ③ Have a variety of cell receptors, can accurately decode signals;
- ④ Rapid degradation, termination of the signal and prevent desensitization;
Neuronal activity is the basis of information encoding and processing in the brain.
During neuron excitation, ATP is produced to meet the high energy demand.
At the same time, ATP can also be secreted into the extracellular, acting as a central neurotransmitter that inhibits the activity of nerve cells to prevent overexcitation of the nervous system.
ATP is released by all brain cells, widely diffuses and targets different types of purinergic receptors on neurons and glial cells, and coordinates the activity of brain neurons, participating in various physiological processes such as sleep and wake, learning and memory, and feeding.
Dysregulation of extracellular ATP can lead to neurological dysfunction, which has been found in the etiological pathology of psychiatric disorders such as depression, anxiety, schizophrenia, and Autism spectrum disorder (ASD).
Recently, Professor Tianming Gao of Southern Medical University/Guangdong-Hong Kong-Macao Greater Bay Area Brain Science and Brain Research Center published a paper entitled “Extracellular ATP Is a Homeostatic” in Biological Psychiatry by John H. Krystal, Academician of the National Academy of Medicine of the United States Messenger That Mediates Cell-Cell Communication in Physiological Processes and Psychiatric Diseases “review article.
The article was co-written by Academician Gao Tianming of Southern Medical University, Professor Chen Yihua of Southern Medical University, Professor Lin Song of Jinan University, and illustrated by Dr. Jin Yuanyang of Southern Medical University.
This paper reviews the recent progress of extracellular ATP as an intercellular signaling molecule regulating neural activity, with emphasis on how ATP maintains the homeostasis of neural networks.
This article summarized the abnormal changes of neural activities caused by the dysregulation of extracellular ATP homeostasis, emphasized the important role of the dysregulation of extracellular ATP homeostasis in the etiology and pathology of some mental diseases, clarified the role and mechanism of extracellular ATP in intercellular communication and mental diseases, and prospected the application prospect of ATP as a potential therapeutic target in the treatment of mental diseases.

Overview of ATP signal
ATP is produced by glycolysis and mitochondrial oxidative phosphorylation and can be released from almost all types of cells in the central nervous system.
ATP can be hydrolyzed by at least four exonuclease enzymes to produce Adenosine diphosphate (ADP), Adenosine monophosphate (AMP), and Adenosine (ADO).
P2XR is a cation channel, including P2X1R ~ P2X7R; P2YR is a Gprotein-coupled receptor (GPCR) consisting of P2Y1R, P2Y2R, P2Y4R, P2Y6R, and P2Y11R-14R.
ATP receptors, also known as P2 receptors (P2Rs), can be pharmacologically divided into two subtypes: P2XR and P2YR.
ADO receptors, also known as P1 receptors, consist of A1R, A2AR, A2BR, and A3R, all of which are GPCRS.
Different ATP receptors show sensitivity to ATP from the nmol level to the mmol level, so ATP can cause a variety of reactions.
ATP receptors can function throughout the central nervous system and produce different effects depending on the receptor of action.
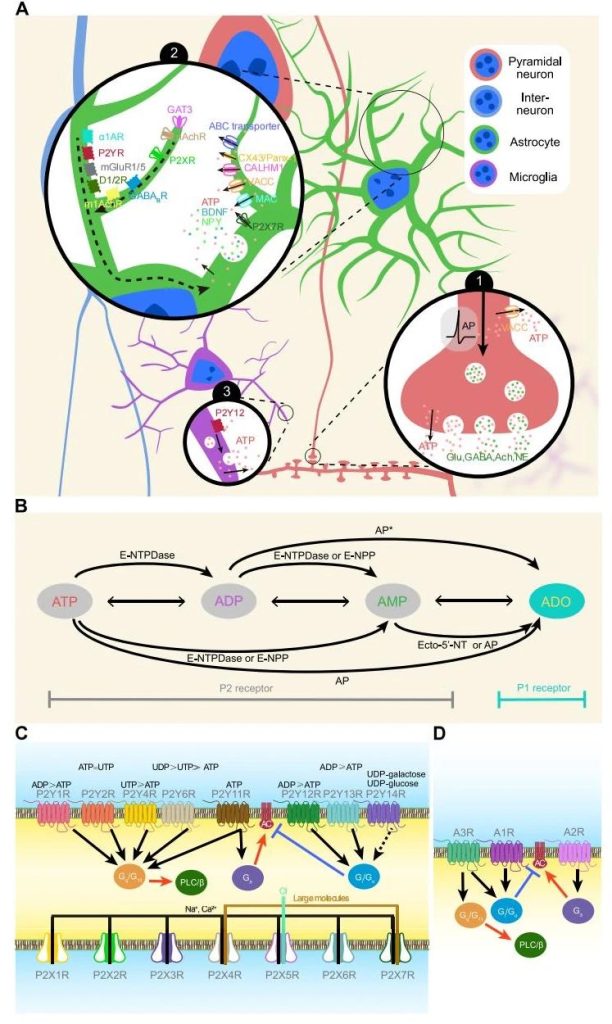
Extracellular ATP acts as an homeostasis messenger to regulate neuronal activity and brain circuits
In 1992, researchers first discovered ATP as a central nervous system neurotransmitter in the medial habenula.
Electron microscopy showed that P2X2R and P2X4R, the two main receptors for rapid response to ATP, were mostly located in the perisynaptic and postsynaptic extracellular sites, that is, far from the presynaptic terminal transmitter release area.
Atp-mediated excitatory postsynaptic currents tend to be small and rare, and can only be detected with strong electrical stimulation of certain subpopulations of neurons in specific brain regions.
At present, the experimental evidence does not support ATP as a fast neurotransmitter, but ATP may be a neuromodulator.
Endogenous ADO has been reported to reduce excitatory synaptic transmission in hippocampal pyramidal neurons by activating the A1 receptor of hippocampal CA1 pyramidal neurons, thereby reducing the excitability of hippocampal CA1 pyramidal neurons, or inhibiting the release of presynaptic glutamate.
In hippocampal CA3, neurons containing P2X2Rs form synapses with inhibitory interneurons and are activated by ATP during theta oscillation, causing the release of glutamate, which in turn excites interneurons and increases the inhibition of neuronal networks.
In the hippocampus, activation of P2X2R increases the activity of phosphatase or CaMKII (calcium/calmodulin-dependent protein kinase II) after calcium influx, internalizing the membrane surface AMPA receptor and causing synaptic inhibition.
P2X7Rs is very rare in neurons, so this mechanism is not widespread in the center.
In the paraventricular nucleus, endogenous ATP activates extra-synaptic P2X7R and increases postsynaptic effects by inserting AMPA receptors into dendritic spines.
Postsynaptic activation of P2Y1R or P2XR in The pyramidal neurons of The prefrontal cortex (PFC) had inhibitory effects on NMDA receptors.
In neural networks, inhibitory synaptic transmission is essential for brain activity.
ATP is involved through the following mechanisms:
- 1) PFC and hippocampal interneuron cell body express P2Y1Rs, and activation of these receptors causes inwardly non-selective cationic current, which depolarizes the interneuron membrane and increases its discharge frequency, resulting in increased synaptic inhibition of pyramidal neurons.
- 2) Extracellular ATP activates P2X2R and promotes the release of presynaptic GABA (gamma-aminobutyric acid).
- 3) Astrocytes respond to the release of endogenous GABA by releasing ATP. Subsequently, ATP is broken down into ADO, which activates post-synaptic A1R and increases synaptic inhibition of pyramidal cells.
- 4) Enhanced inhibitory transmission may also be due to enhanced GABAA receptor function in postsynaptic neurons.
Notably, extracellular ATP can also reduce neuronal activity in a circuit-dependent manner.
For example, extracellular ATP induces The activation of GABaergic interneurons via P2X2R in the PFC, thereby inhibiting the activity of the medial PFC (mPFC) – The lateral habenula (LHb) circuit.
In the amygdala, extracellular ATP inhibits excitatory synaptic transmission to the basolateral amygdala and increases inhibitory synaptic transmission to the lateral central amygdala by activating A1R and A2AR via adenosine, respectively.
The initial axon segment is the site where the action potential is generated and is susceptible to external signals.
One study showed that extracellular ATP inhibits the production of action potentials in highly active neurons by acting on P2X7R in the initial segment of the axon.
Astrocyte derived ATP may also regulate signaling along axons, and at Ranvier’s nodes, its hydrolyzed product ADO slows axon conduction via A2R.
ATP release from astrocytes is regulated by synaptic activity, and synaptic activation can inhibit neuronal activity through paracrine and autocrine ATP, while ATP release from surrounding cells can increase astrocyte [Ca2+]i, further triggering ATP release and forming a positive feedback. These feedback mechanisms may work together to suppress local loops.
ATP/ADP released by microglia can activate P2Y12R of microglia, resulting in a rapid increase in the contact site between the neuronal cell body and the microglia process to prevent excitatory toxicity.
Consistent with these findings, microglia-specific knockout of P2Y12R increased the excitability of CA1 pyramidal neurons.
Microglia can hydrolyze ATP to ADO and further activate A1R.
A1R is expressed at the presynaptic site, and activation of A1R reduces neurotransmitter release and neuronal excitability in a G-protein-dependent manner.
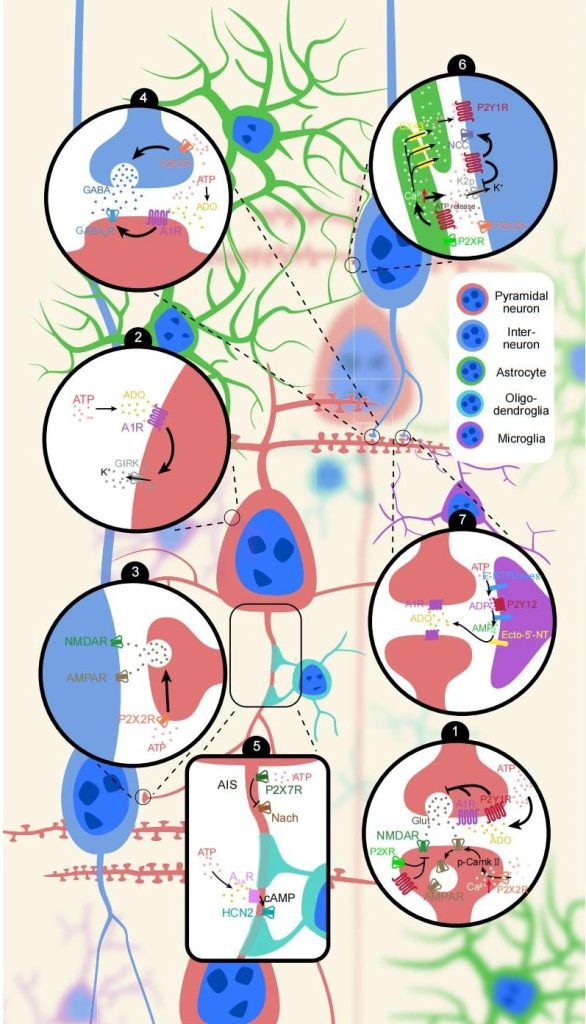
Physiological role of extracellular ATP
Extracellular ATP plays a key role in coordinating neuronal activity in the brain and is involved in a variety of physiological processes, including sleep and wake, learning and memory, and feeding.
1. Sleep and awakening
The basal forebrain (BF) plays a crucial role in sleep-wake regulation.
The study found that ATP levels in BF are stable during wakefulness, but surge during sleep and correlate with delta activity during Nonrapid eye movement (NREM) sleep.
Other studies have found that extracellular ATP levels increase during awake and Rapid-eye movement (REM) sleep and decrease during NREM sleep, and its function may be related to promoting wakefulness.
Supporting this idea is that activation of P2R in BF promotes arousal, while inhibition of P2R promotes sleep.
ADO inhibits glutamatergic input from GABaergic neurons to promote sleep.
Extracellular ATP depolarizes GABAergic neurons in BF and plays a role in promoting arousal.
These observations provide important insights into the role and mechanisms of extracellular ATP in homeostasis regulation of sleep and wakefulness.
2. Learning and memory
Extracellular ATP signaling significantly affects learning and memory.
For example, the lack of ATP release or the absence of receptors in astrocytes leads to impairments in learning and memory.
Synaptic plasticity is the basis of learning and memory, including Long-term potentiation (LTP) and Long-term depression (LTD).
It is well known that extracellular ATP signaling has significant effects on both LTP and LTD.
For example, local administration of ATP can cause small or large amounts of Ca2+ to flow into hippocampal CA1 neurons, leading to LTP or LTD, respectively.
Lack of ATP release by astrocytes impairs LTP induction.
P2X3R knockout mice showed impaired LTD, but no effect on LTP.
In contrast, P2X4R knockout mice showed LTP-induced impairment.
Activation of P2YR attenuates glutamate release from hippocampal CA1 neurons, leading to induction of heterosynaptic LTD.
These findings highlight the role of extracellular ATP signaling in regulating learning and memory and hippocampal synaptic plasticity.
3. ingestion
The hypothalamus, a complex structure of small nuclei such as the arcuate nucleus of the thalamus, the lateral nucleus of the hypothalamus, and the paraventricular nucleus, is a recognized brain region involved in the control of eating.
Anatomically, P2XRs is heavily expressed in the arciform nucleus, paraventricular nucleus, and other hypothalamic nuclei, while P2Y1Rs is expressed in the ventromedial and lateral hypothalamic nuclei, suggesting that ATP may be involved in the regulation of feeding.
Functionally, ATP treatment in the hypothalamic region of the brain slices induced excitatory responses in neurons of the lateral hypothalamus, paraventricular nucleus, doromedial nucleus of hypothalamus, and ventromedial nucleus of hypothalamus.
In summary, ATP seems to have excitatory effects on hypothalamic neurons.
To date, only a few studies have directly shown how extracellular ATP affects feeding.
Intraventricular injection of ATP/ADP analogs increased rat food intake, which could be blocked by P2Y1R selective antagonist preconditioning, while pharmacological activation of P2Y1R in the ventromedial and lateral hypothalamic nuclei enhanced food intake.
Non-selective pharmacological inhibition of P2 receptors in the nucleus accumbens reduces food intake and eating time.
higher-weight person mice with restricted inactivation of P2Y6 receptors in Agouti-related peptide (AgRP) neurons showed reduced food intake.
A recent study showed that photogenetic activation of tantan cells in the hypothalamus can cause acute overeating by activating purinergic receptors on orexigenic neuropeptid-positive neurons in the arcuate nucleus.
The specific role and underlying mechanisms of P2R in hypothalamic feeding regulation of different cell types are unclear.
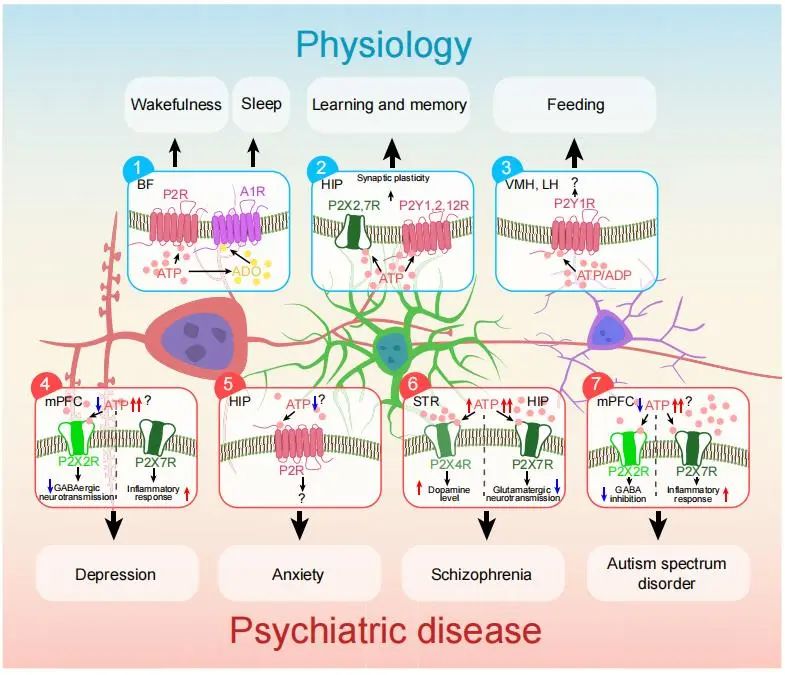
The role of dysregulation of extracellular ATP in the pathogenesis of psychiatric diseases
A large number of studies have shown that the physiological processes of various behaviors require the participation of extracellular ATP, and the dysfunction of extracellular ATP is related to the etiology and pathology of many mental diseases, including depression, anxiety, schizophrenia and autism spectrum disorder (ASD).
1. depressed
The relationship between extracellular ATP and the pathogenesis and successful treatment of Major depressive disorder (MDD) has been extensively studied.
Previous studies have shown reduced ATP synthesis in the Basal ganglia and Frontal cortex in MDD patients.
Further research showed that MDD patients who committed killed themself had changes in the expression of genes associated with ATP biosynthesis and utilization in the PFC.
In addition, effective MDD treatment correlates with normal levels of ATP biosynthesis.
Studies in rodents have found that ketamine, a clinical fast-acting antidepressant, produces a rapid antidepressant effect accompanied by increased ATP levels.
These studies suggest that the reversal of depressive symptoms is associated with the restoration of ATP-related bioenergetic states.
Studies in animal models of depression have shown that reduced levels of extracellular ATP in the PFC and hippocampus inhibit ATP release from astrocytes, leading to depression-like behavior in mice.
In contrast, enhancing ATP release from astrocytes has an antidepressant effect that is blocked by the enzyme adenosine triphosphate diphosphonase in mPFC.
Recent studies have shown that reducing the release of ATP reduces the stimulation of P2X2R, thereby reducing the excitability of GABAergic interneurons in mPFC, thereby reducing the inhibitory effect of GABaergic neurons on mPFC neurons that project to LHb, enhancing the activity of this neural circuit and inducing depression-like behaviors.
Extracellular ATP concentration affected by its release and enzymatic degradation.
Recent studies have confirmed the effect of ATP release and degradation on depression.
For example, astrocyte epoxyeicosatrienoic acid signaling and glucocorticoid receptors involved in regulating exocytodependent ATP release and expression of depression-like behavior.
Calhm2, a non-extracellular ATP releasing factor, also plays a role in depressive symptoms.
With respect to enzymatic degradation of ATP, upregulation of nucleotide tri (ii) phosphohydrolase 1 (hydrolyzed extracellular ATP) in the hippocampus associated with social avoidance and desperate behavior, but not with anhedonia induced by chronic social frustration stress.
Together, these findings advance our understanding of the complex role of ATP release and enzyme degradation in depression.
P2X7R activated by high ATP concentration (EC50≥100 mmol/L) during immune activation and tissue damage, which can effectively trigger inflammasome activation, which may involved in the pathogenesis of depression.
Notably, some studies have shown that binding stress can lead to a temporary increase in extracellular ATP concentration.
P2X7R knockout mice showed an antidepressant like phenotype and increased resilience to stress.
Whether extracellular ATP accumulation can activate P2X7R in these depressed animals remains to further investigated.
2. anxiety
Research on the role of extracellular ATP signaling in anxiety is increasingly common.
P2X7R with a specific single nucleotide polymorphism (rs1718119) associated with an increased risk of anxiety attacks in people with anxiety disorders.
Some studies have failed to find the effect of P2X7R inactivation on anxiety-like behavior in mice.
This difference may attributed to the presence of active P2X7R splicing variants in the knockout mouse models employed, allowing them to escape gene inactivation.
Conversely, studies have shown that ATP signaling also has anti-anxiety effects.
A recent study used a transgenic mouse model with increased P2X4R surface density in the hippocampus, which showed anti-anxiety effects.
Non-specific P2YR agonists produce antianxiety effects, whereas P2Y12R knockout produces anxiety-like effects.
Photogenetic activation of hippocampal astrocytes induces ATP release and produces antianxiety-like behavior that can blocked by non-specific P2R antagonists.
3. schizophrenia
Schizophrenia has a variety of symptoms, including reduced emotional responsiveness, impaired social cognition, and cognitive deficits.
Studies have linked elevated ATP signaling to the development of schizophrenia, with higher levels of ATP biosynthesis observed in the left hemisphere of schizophrenia patients.
Recent reports have also shown increased mRNA levels of P2X7R in the dorsolateral PFC in patients with schizophrenia, which correlates with levels of the inflammatory marker SERPINA3.
In rodents, blocking P2X7R through drugs and genetic methods can alleviate schizoid behavior.
Notably, P2X7R plays a key role in regulating hippocampal excitatory neurotransmission.
Given the role of glutaminergic neurotransmission in the pathophysiology of schizophrenia, these findings suggest a possible link between P2x7R-mediated inflammatory signaling, glutaminergic neurotransmission and the pathogenesis of schizophrenia.
The proximity of the P2X7R and P2X4R genes on human chromosome 12 reflects a close relationship between the two, suggesting that P2X4R may also play a role in schizophrenia.
Researchers have observed increased mRNA levels of P2X4R in dorsolateral PFC in patients with schizophrenia.
Notably, both pre – and post-synaptic dopaminergic markers altered in the striatum of P2X4R knockout mice, suggesting that P2X4R plays an important role in maintaining dopamine homeostasis.
Dopamine receptor antagonists reduce schizoid behavior induced by activation of P2X4R.
Since hyperactivity of the dopamine system associated with schizophrenia, these findings suggest that activation of P2X4R may contribute to the progression of schizophrenia to some extent by inducing hyperactivity of the dopaminergic system.
Given that the P2X7R and P2X4R genes are closely adjacent, the two receptors may activated by high levels of ATP when they function as P2X4-P2X7R multiprotein complexes.
There is evidence that the application of a selective P2Y1R agonist to mPFC induces schizoid behavior, while activation of P2Y1R increases dopamine release in NAc (Nucleus accumbens).
More studies needed in the future to determine the relationship between P2Y1R, dopamine activity and schizophrenia.
4. Autism spectrum disorder
ASD characterized by difficulties in social interaction and communication, and accompanied by limited and repetitive behaviors.
Currently, there are few clinical studies on the role of extracellular ATP in ASD, but current animal studies have hinted at the involvement of ATP.
Our recent research has shown that impaired Ca2+ signaling in astrocytes can lead to ASD-like behaviors, including impaired social interaction and repetitive behaviors.
ATP administration reversed impairments in social interaction without affecting repetitive behaviors, suggesting that dysfunctions of extracellular ATP signaling may primarily affect social interaction.
Another study found that the ablation of the P2X4R gene resulted in a disturbance of social communication function, which just confirmed the above conjecture.
Recent studies have shown that repetitive behavioral deficits may caused by impaired Ca2+ signaling in astrocytes in the striatum, and whether ATP signaling involved needs to further explored.
Elevated levels of extracellular ATP may act as a “pathogen,” triggering an inflammatory response that can lead to ASD.
For example, high levels of ATP activate P2X7R and induce ASD-like behavior in mice, and inflammatory signaling mediated by P2X7R activation has also implicated in animal models of autism.
Together, these studies suggest that disruption of extracellular ATP homeostasis may involved in the pathogenesis of ASD and depression.
In fact, ASD and depression do have a high comorbidity rate.
The role of ATP signaling in the treatment of psychiatric disorders
The authors discuss the critical role of ATP signaling in psychiatric disorders, highlighting ATP and its receptors as potential therapeutic targets.
In fact, our team and other researchers have shown that increasing extracellular ATP levels can improve depression-like and autism-like behaviors.
Not only that, other researchers have found that an exonucleotidase inhibitor, ARL67156, alleviates depression-like behavior by reducing extracellular metabolism of ATP.
In addition, both the soluble cyclooxygenase inhibitor TPPU and caloric restriction can produce an antidepressant like effect, which may achieved by increasing ATP release from astrocytes.
Among P2Rs, P2X7R is a promising target for psychiatric disorders because it can specifically activated by unusually high extracellular ATP levels, which ensures that its blocking does not affect the effects of physiological ATP release acting on other subtypes of P2XR.
P2X7R antagonists include Abbott’s A-804598 and A-438079 and Janssen’s JNJ-47965567;
These antagonists have shown to improve depression and anxiety behavior in rodents.
A clinical study reported that JNJ-54175446(from Janssen) can reduce the anhedonia that occurs after complete sleep deprivation in patients with major depression.
JNJ-47965567 also improved schizoid and autism-like behaviors.
Clinical trials are also underway.
Of note, a Phase II double-blind, placebo-controlled clinical study designed to evaluate the safety and efficacy of ATP in combination with fluoxetine for rapid improvement in depression (NCT05431413).
In addition, two clinical studies are evaluating the efficacy of P2X7R antagonists JNJ-54175446 (NCT04116606) and JNJ-55308942 (NCT05328297) in the treatment of depression.
A small sample Phase I and II clinical trial in children with ASD is underway to evaluate the anti-ASD effect of suramin, a non-specific P2R antagonist (NCT02508259).
The results showed that children with ASD who received low doses of suramin showed significant improvement in symptoms.
Despite these advances, more research, including larger clinical trials, needed to clarify the potential of ATP signal-related therapies in the treatment of psychiatric disorders.
conclusion
In summary, there is credible evidence that ATP released by neurons, astrocytes, and microglia can activate P2R, and that ADO produced by ATP breakdown can activate P1R in different regions of the central nervous system.
Atp-mediated signaling has less effect on postsynaptic membrane depolarization, but it contributes to slow and diffuse regulation of synaptic homeostasis and plasticity.
ATP can inhibit neural networks in different physiological environments.
This impairment of signaling has linked to the occurrence of several psychiatric disorders.
Although some progress has made in this area, the following points still need to further explored.
- 1) Due to different concentrations, extracellular ATP may have different or even contradictory effects. The exact mechanism of ATP homeostasis regulation remains to further explored.
- 2) In view of the importance of extracellular ATP concentration, it is necessary to monitor ATP concentration and its degradation product ADO in real time under physiological and pathological conditions. The development of tools to specifically identify ATP and ADO, for example, genetically encoded fluorescent indicators, will provide new directions in this field.
- 3) The specific functions of purinergic receptors in different cell subtypes in physiological and pathological contexts need to better understood to provide a basis for drug intervention. Fortunately, the emergence of new technologies is driving progress in this area.
The original link: DOI: 10.1016 / j.b iopsych. 2024.04.013
reference
- [1] Fiske C H, Subbarow Y.PHOSPHORUS COMPOUNDS OF MUSCLE AND LIVER[J]. Science, 1929, 70(1816):381-382.
- [2] DiVirgilio F, Vultaggio-Poma V, Falzoni S, et al. Extracellular ATP: A powerful inflammatory mediator in the central nervous system[J]. Neuropharmacology, 2023,224: 109333.
- [3] BurnstockG, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor?[J]. Gen Pharmacol, 1985, 16(5): 433-440.
- [4]ZimmermannH. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system[J]. Prog Neurobiol, 1996, 49(6): 589-618.
- [5]Lalo U, Pankratov Y. ATP-mediated signalling in the central synapses[J]. Neuropharmacology, 2023, 229: 109477.
- [6]Peng W, Liu X, Ma G, et al. Adenosine-independent regulation of the sleep-wake cycle by astrocyte activity[J]. Cell Discov,2023, 9(1): 16.
- [7]YangC, Larin A, McKenna J T, et al. Activation of basal forebrain purinergic P2 receptors promotes wakefulness in mice[J]. Sci Rep, 2018, 8(1): 10730.
- [8]Chi S, Cui Y, Wang H, et al. Astrocytic Piezo1-mediated mechanotransduction determines adult neurogenesis and cognitive functions[J]. Neuron, 2022, 110(18):2984-2999.e2988.
- [9]Domingos L B, Hott S C, Terzian A L B, et al. P2X7 purinergic receptors participate in the expression and extinction processes of contextual fear conditioning memory in mice[J]. Neuropharmacology, 2018, 128: 474-481.
- [10]Kittner H, Franke H, Harsch J I, et al. Enhanced food intake after stimulation of hypothalamic P2Y1 receptors in rats: modulation of feeding behaviour by extracellular nucleotides[J]. Eur J Neurosci, 2006, 24(7):2049-2056.
- [11]Kittner H, Krügel U, Hoffmann E, et al. Modulation of feeding behaviour by blocking purinergic receptors in the rat nucleus accumbens: a combined microdialysis, electroencephalographic and behavioural study[J]. Eur J Neurosci, 2004,19(2): 396-404.
- [12]Steculorum S M, Timper K, Engström Ruud L, et al. Inhibition of P2Y6 Signaling in AgRP Neurons Reduces Food Intake and Improves Systemic Insulin Sensitivity in Obesity[J]. Cell Rep, 2017, 18(7): 1587-1597.
- [13]Cao X, Li L P, Wang Q, et al. Astrocyte-derived ATP modulates depressive-like behaviors[J]. Nat Med, 2013, 19(6): 773-777.
- [14]Lin S, Huang L, Luo Z C, et al. The ATP Level in the Medial Prefrontal Cortex Regulates Depressive-like Behavior via the Medial Prefrontal Cortex-Lateral Habenula Pathway[J]. Biol Psychiatry,2022, 92(3): 179-192.
- [15]Erhardt A, Lucae S, Unschuld P G, et al. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders[J]. JAffect Disord, 2007, 101(1-3):159-168.
- [16]Basso A M, Bratcher N A, Harris R R, et al. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders[J]. Behav Brain Res, 2009, 198(1): 83-90.
- [17]Deicken R F, Calabrese G, Merrin E L, et al. Asymmetry of temporal lobe phosphorous metabolism in schizophrenia: a 31phosphorous magnetic resonance spectroscopic imaging study[J]. Biol Psychiatry, 1995, 38(5): 279-286.
- [18]Trubetskoy V, Pardiñas A F, Qi T, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia[J]. Nature,2022, 604(7906): 502-508.
- [19]WangQ, Kong Y, Wu D Y, et al. Impaired calcium signaling in astrocytes modulatesautism spectrum disorder-like behaviors in mice[J]. Nat Commun, 2021, 12(1):3321.