What is glutathione?
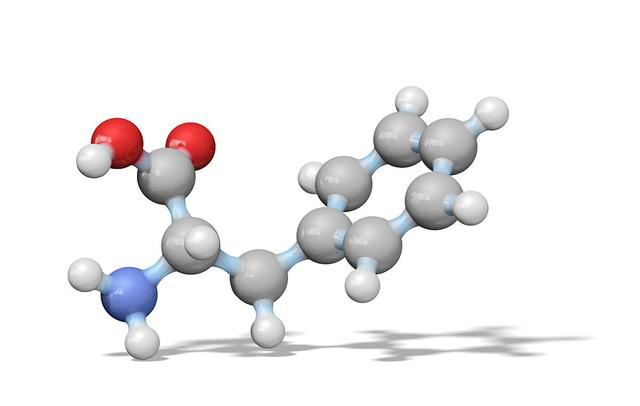
Glutathione (GSH), also known as reduced glutathione, is a kind of active tripeptide with important physiological functions. It is formed by the condensation of glutamic acid, cysteine and glycine by peptide bond. The cysteine side chain group is connected with an active sulfhydryl group (-SH), which is a kind of efficient antioxidant.
The content of reduced glutathione is generally higher in animal liver, wheat germ and yeast.
Glutathione functions in the following ways:
1, Protect biological proteins
GSH can restore the activity of sulfhydryl groups in the enzyme molecules that have been destroyed, and prevent the degeneration of proteins (such as hemoglobin) caused by the oxidation of sulfhydryl groups.
2, remove free radicals in the body
Under the catalysis of glutathione peroxidase (GSH-PX), GSH can reduce the H2O2 produced in the cell to O2 and H2O, and GSH oxidized to oxidized glutathione (GSSG), which catalyzed by glutathione reductase to regenerate GSH.
Through this reaction, GSH can remove oxygen ions and other free radicals in the body, inhibit lipid peroxidation, reduce melanin formation and skin aging, and maintain the integrity of erythrocyte membrane. Reduce free radical attack on DNA, thereby reducing DNA damage and mutation.
3, Neutralize and detoxify
GSH can combined with various toxic compounds, heavy metal ions and other harmful substances that invade the body of external organisms, and promote their excretion, and play a role of neutralization and detoxification.
4, Protect white blood cells
GSH can prevent leukopenia caused by radiation, radiopharmaceutics and antitumor drugs.
What is glutathione peroxidase?
Glutathione peroxidase (GSH-Px) is the first selenium-containing enzyme found in mammals.
Selenium is the active component of glutathione peroxidase and an essential component of GPX-Px catalytic reaction. It acts in the form of selenocysteine, and the activity of GSH-Px enzyme decreases when selenium is insufficient.
When the body at a low selenium level, the enzyme activity positively correlated with the intake of selenium, but at a certain level, the enzyme activity no longer increases with the increase of selenium level.
Gsh-px exists in the cellular fluid and mitochondrial matrix, it uses glutathione (GSH) as a reducing agent to decompose peroxides in the body, can reduce toxic peroxides to non-toxic hydroxyl compounds, and decompose hydrogen peroxide into alcohol and water, so it can prevent cell membranes and other biological tissues from damaged by peroxide.
It, together with superoxide dismutase (SOD) and catalase (CAT) in the body, constitutes an antioxidant defense system, so it plays an important role in the body’s antioxidant.
Under normal conditions, most of the reactive oxygen species cleared by the body’s defense system, but when the body produces some lesions, excess reactive oxygen species will destroy the cell membrane.
The body’s first line of defense against reactive oxygen species O2-· is superoxide dismutase (SOD), which converts O2-· to hydrogen peroxide and water, while the second line of defense is catalase and GSH-Px.
CAT can remove H2O2, while GSH-Px distributed in the cytosol and mitochondria of cells, eliminating H2O2 and hydroperoxide.
GSH-Px, SOD and CAT work together to eliminate active oxygen species and reduce and prevent lipid peroxidation.
GSH-Px exists widely in mammalian tissues, and the molecular weight and specific activity of different kinds of GSH-Px are also different.
Glutathione is the specific and specific substrate of this enzyme, while hydroperoxide is the non-specific substrate.