Professor Kang Zhen research group of Academician Chen Jian in the School of Biological Engineering has made important progress in the efficient synthesis of controlled molecular weight of hyaluronic acid based on cell metabolism and morphological regulation.
The results “regulate cellular metabolism and morphology to achieve high-yield synthesis of hyaluronan with controllable. molecular weights “is published in Nature Communications (IF = 14.7).
Hyaluronic acid (HA) is a high-value glycosaminoglycan widely used in daily life. The efficient biosynthesis and precise molecular weight control of HA are current research difficulties, mainly limited by the insufficient functional analysis of type I hyaluronic acid synthase (HAS) and the imbalance of cell metabolism and morphology.
In this study, by systematically characterizing the key functional regions of HAS, we constructed mutants that can achieve complete HA secretion and extend the molecular weight range to 300-1400 kDa.
Through dynamic regulation of UDP-glucose-6-dehydrogenase activity combined with adaptive domestication strategy, normal cell growth was restored and metabolic capacity was improved.
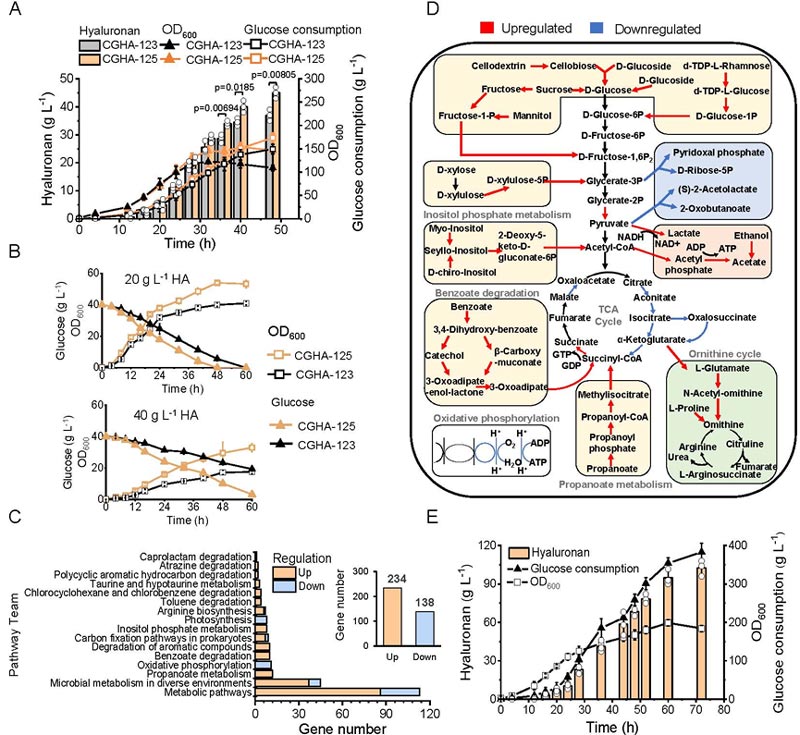
Finally, high and medium molecular weight HA (500 kDa) and low molecular weight HA (10 kDa) reached 45 g·L−1 and 105 g·L−1, respectively, with production efficiencies of 0.94 g·L−1·h−1 and 1.46 g·L−1·h−1.
This study deepened the understanding of HAS function and the mechanism of cell metabolism-morphology interaction, and provided a model for morpho-oriented engineering strategies in microbial cell factories.
Type I HAS is a multifunctional transmembrane protein whose cytoplasmic domain is responsible for substrate binding and HA polymerization, and transmembrane channels regulate HA chain extension and transport.
After the exogenous introduction of Streptococcus epizooticus HAS (SzHAS) into Corynebacterium glutamate, it was found that SzHAS retained HA polymerization activity, but the abnormal conformation of transmembrane channels resulted in the intracellular accumulation of HA, resulting in cell morphological distortion, membrane damage and growth and metabolism arrest.
Lipidomics analysis showed that the phosphatidylinositol (PI) content of Corynebacterium glutamicum was significantly lower than that of Streptococcus epizoosis.
After PI synthase overexpression increased PI level, HA remained in the cell, but the cell growth was partially restored, and the morphology and membrane integrity were significantly improved.
Studies have shown that conformation of SzHAS transmembrane channels regulated by the membrane lipid environment, and its correct conformation plays a key role in the regulation of HA biosynthesis and transport.
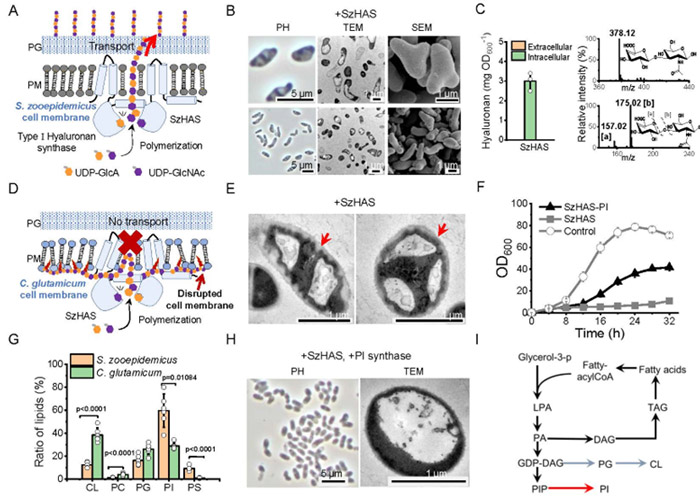
The structure and amino acid sequence of Streptococcus pyogenes derived from HAS (SpHAS) are highly similar to that of SzHAS, but in Corynebacterium glutamate, SpHAS can synthesize and secrete HA, indicating that the difference of HAS sequence has an important effect on its conformation and function.
In order to analyze the key domain, SzHAS transmembrane helical TMH-1 replaced with the corresponding fragment of SpHAS, and the mutant SzHASSpTMH-1 restored the ability of HA secretion.
The AlphaFold2 structural simulation showed that TMH-1 not directly involved in the construction of transmembrane channels, and its absence did not affect polymerization activity but regulated HA secretion.
Studies have shown that TMH-1 regulates the conformation of transmembrane channels by interacting with TMH-2, thus affecting HA transport.
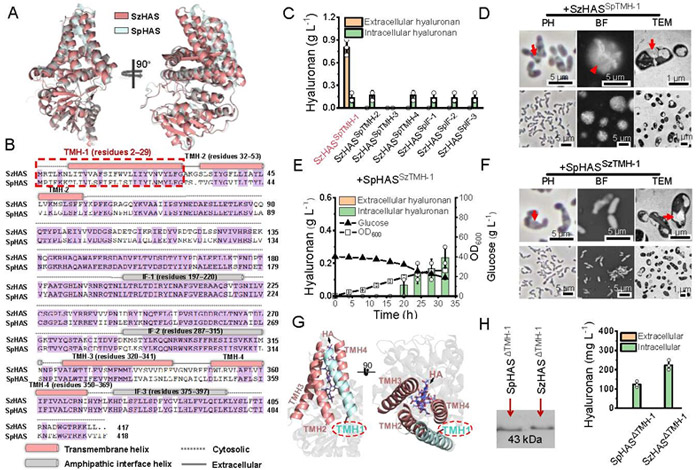
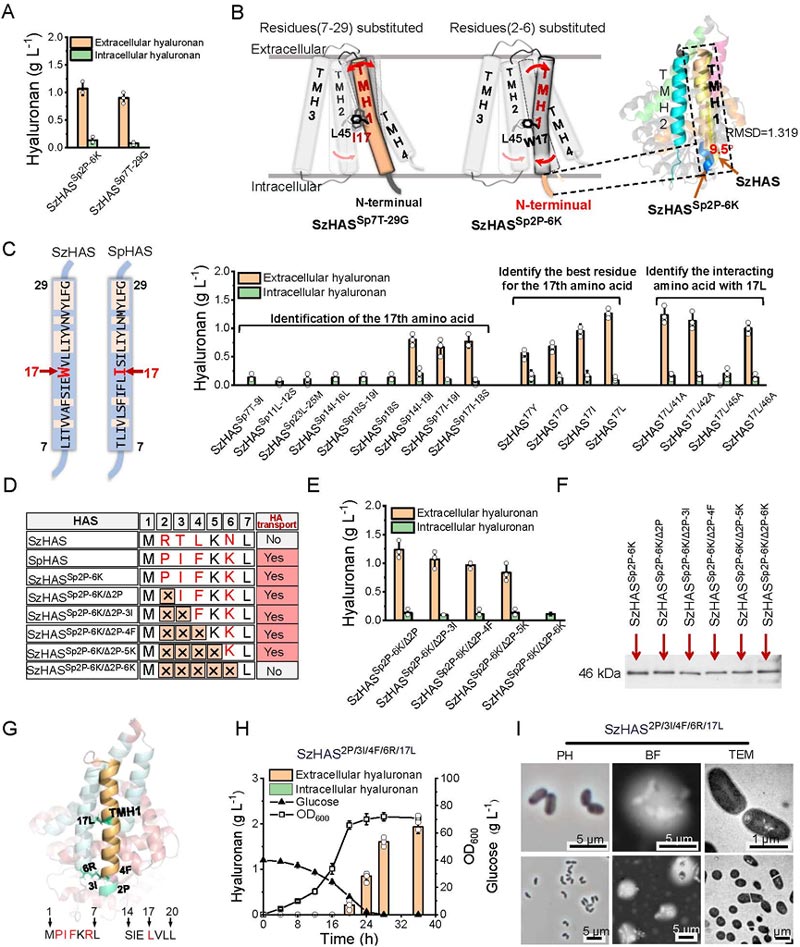
The authors systematically identified the key amino acid sites of TMH-1 regulating HA transport, and found that TMH-1 residues 17 and N-terminal residues 2, 3, 4 and 6 were essential for HA transport through truncation and site-specific mutation.
Studies have shown that W17 in TMH-1 and L45 in TMH-2 synergically regulate transmembrane channel conformation through non-covalent interaction, and L45 mutation can completely block HA transport.
The optimal mutant SzHAS2P/3I/4F/6R/17L obtained by integrating beneficial mutations, and its HA synthesis efficiency was 30% higher than that of SpHAS.
The conformation state of transmembrane channels significantly affects the degree of HA polymerization, which may be related to the intensity of channel-new chain interaction.
For example, the elongation and release delay of HA chains in the semi-open state lead to the increase of molecular weight, and finally the controlled synthesis of HA molecular weight between 300 and 1400 kDa achieved.
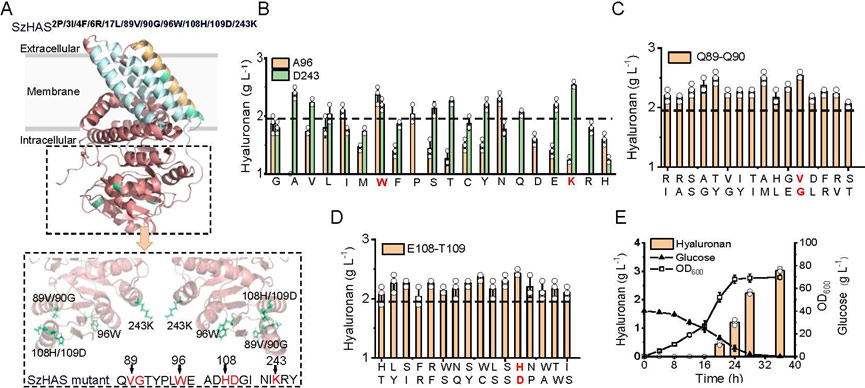
Based on structural prediction, systematic mutation screening conducted for 12 non-conserved sites in the SzHAS catalytic domain, and it found that mutations in Q89V/Q90G, A96W, E108H/T109D and D243K could significantly increase HA yield.
The mutant strains SzHAS2P / 3 I/f / 4 6 r/build mutant SzHAS2P 17 l / 3 I/f / 4 6 r / 17 l / 89 v / 96 w / 90 g / 108 h / 109 d / 243 k, the synthesis ability significantly enhanced HA, upgrade 50% base mutant strains.
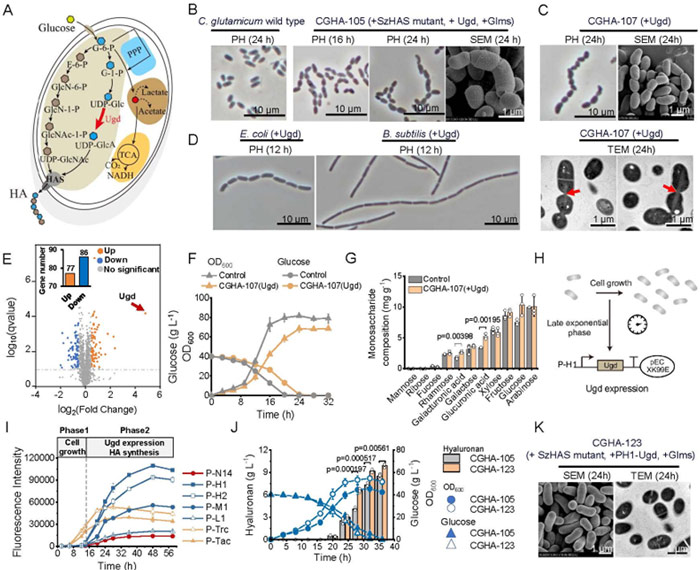
Strengthening the UDP-glucose dehydrogenase gene ugd, which is the pathway of HA precursor synthesis, can increase the supply of UDP-glucuronic acid and significantly enhance the synthesis capacity of HA.
Overexpression of ugd leads to rod-chain transformation of Corynebacterium glutamicum and other hosts (E. coli, Bacillus subtilis).
Transmission electron microscopy (TEM) analysis showed that the chain morphology was due to incomplete cell division.
The analysis of cell sugar composition showed that the content of polysaccharide containing glucuronic acid in cell wall abnormally increased, suggesting that Ugd overexpression changed the cell wall composition and affected cell division.
The synthesis of HA mainly concentrated in the late logarithmic growth phase, while the cell morphological changes occurred in the early logarithmic growth phase.
In order to coordinate cell growth and product synthesis, we adopted an exponential late responsive promoter, and obtained a high-strength promoter PH1 through promoter engineering to dynamically regulate the expression of UDP-glucose dehydrogenase, significantly improve the production of HA flask, while maintaining normal cell morphology and high density growth.
In the 5 L fermenter fed-batch culture, the increased viscosity of the culture medium resulted in a sharp decrease in metabolic activity of the strain after 36 h.
Based on the adaptive domestication strategy, the resistant strains screened through 300 successive generations in a high-concentration HA environment.
Transcriptomic analysis showed that energy metabolism and stress response pathways significantly up-regulated, suggesting that metabolic flux redistribution and enhanced NAD+ regeneration were key factors for strain tolerance to high-viscosity anaerobic environments.
Finally, the production of HA of medium and high molecular weight was 45 g·L−1.
In order to break through the viscosity restriction, the hyaluronidase derived from leech added at 16 h of feed culture to eliminate the HA capsule layer and improve the glucose uptake efficiency, so that the yield of low molecular weight HA reached 105 g·L−1.
The above work published in Nature Communications.
The School of Bioengineering of Jiangnan University is the first completed unit, and the participating units include Yixing Institute of Food and Biotechnology and South China University of Technology. The first author is Hu Litao, PhD student of Jiangnan University in 2020.
Fayin Wang, undergraduate student of Grade 2022, and Professor Zhen Kang, School of Biological Engineering, Jiangnan University, are the corresponding authors.
The above work has supported by National Key Research and Development Program (2024YFF1106300), National Natural Science Foundation of China (U24A20368), Jiangsu Basic Research Center of Synthetic Biology (BK20233003), State Key Laboratory of Food Science Research Program (BK20233003), and Wuxi Industrial Innovation Research Institute Supported by Research Program (XD24006) and State Key Laboratory of Food Science and Resources Research Program (SKLF-ZZB-202418) of Jiangnan University.
In recent years, Professor Kang Zhen’s team has carried out systematic work on the microbial synthesis of glycosaminoglycans (hyaluronic acid, chondroitin sulfate, heparin, etc.), the mining and expression of related degrading enzymes, and the development of microbial synthetic biology tools, and has achieved a series of original research results.
Some of the results have published in Nature Communications (2023,2020), ACS Catalysis (2021), Green Chemistry (2022,2021), Carbohydrate Polymers (2024, 2022, 2019, 2019) and other authoritative journals in the field.
Hyaluronic acid