Background introduction
Boosting intracellular glutathione levels through its precursors, such as n-acetylcysteine (NAC), is a potential strategy to mitigate oxidative lung damage.
Glutathione (GSH) is a very important and universal antioxidant that reduces inflammatory conditions such as acute respiratory distress syndrome (ARDS)/acute lung injury (ALI).
NAC is a mercaptan molecule with sulfhydryl group that acts as a reactive oxygen species (ROS) scavenger to influence REDOX response, regulate gene expression and cellular inflammatory response.
NAC reduces fibrin in lung tissue, enhances GSH in red blood cells, and reduces the morbidity associated with ALI/ARDS.
NAC’s bioavailability and stability are limited due to its oxidizable sulfhydryl group.
Oral NAC has a bioavailability of 6% to 10%.
Intravenous administration causes disulfide bonds to form between NAC and serum albumin, reducing its bioavailability.
Article highlights
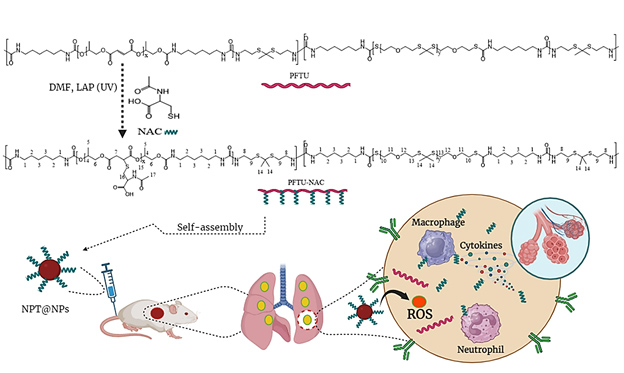
- The NAC grafted polymer nanoparticle delivery system (NPT@NPs) is prepared from a NAC grafted ROS responsive polymer (PFTU), which is synthesized by the click reaction of mercaptan groups in NAC with unsaturated double bonds in PFTU;
- The therapeutic effect of NPT@NPs on lipopolysaccharide (LPS) -induced ALI was systematically analyzed by quantitative characterization of proinflammatory cytokines, pulmonary edema, myeloperoxidase (MPO) expression, and lung cell apoptosis in vivo.
Graphic interpretation
In this study, NAC was grafted to polyurethane (PFTU) synthesized from poly (allyl fumarate), poly (thionone) and 1, 6-hexamethylenediisocyanate to reduce excessive oxidative stress and inflammatory factors in ALI.
A drug delivery system prepared with NAC grafted polymer nanoparticles (NPT@NPs) can effectively scavenge free radicals and reduce inflammation.
In LPS-induced ALI models, NPT@NPs showed significant efficacy in improving pulmonary edema, reducing inflammatory cells, inhibiting MPO expression, reducing pro-inflammatory cytokine levels, and reversing apoptosis.
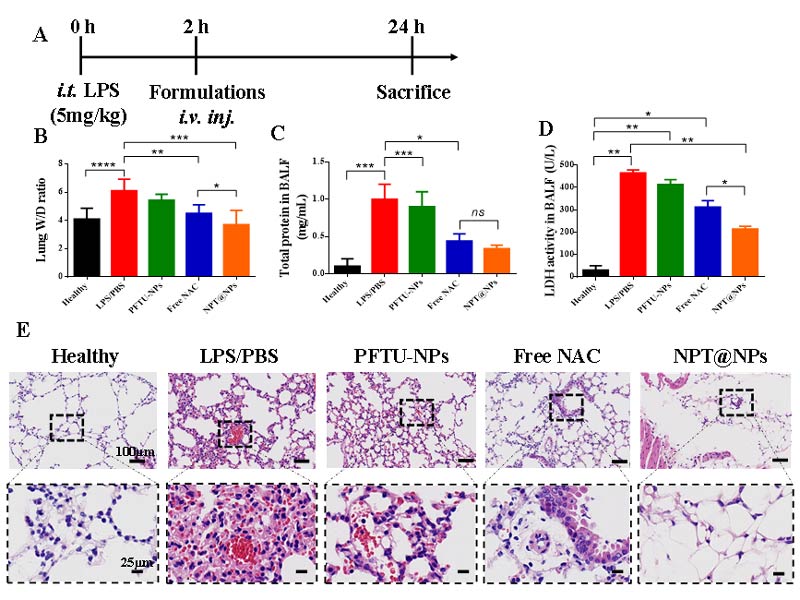
Compared with the other groups, NPT@NPs significantly reduced LPS-induced pulmonary edema.
Compared with LPS group, NPT@NP decreased the total protein concentration.
The study of LDH activity in BALF showed that the injury degree of NPT@NPs group the lowest compared with the control group.
And H&E staining showed that NPT@NPs has a good therapeutic effect.
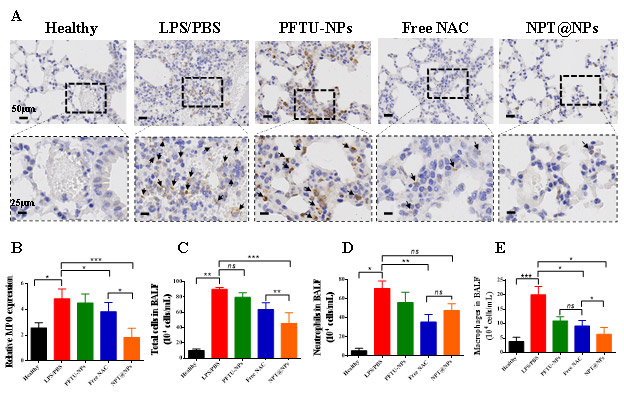
LPS treatment increased MPO activity and showed neutrophil infiltration.
After treatment with NAC, the amount of MPO significantly reduced. PFTU-NP alone also has some effect.
In contrast, NPT@NP significantly reduced MPO levels.
LPS caused significant increases in total cells, macrophages, and neutrophils compared to the control group.
PFTU-NP alone reduced the number of these cells compared to the LPS-treated group.
Free NAC reduces the number of these cells more effectively.
In contrast, NPT@NPs significantly reduced the number of these inflammatory cells.
Summary and prospect
NAC grafted polymer nanoparticles (NPT@NPs) prepared by the click reaction between mercaptan groups in NAC and unsaturated double bonds in PFTU.
NPT@NPs Enhances antioxidant activity by rapidly removing ROS from the ALI microenvironment.
In a mouse model of LPS-induced ALI, the NPT@NPs treatment group showed significantly reduced secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6.
NPT@NPs reduced the number of inflammatory cells, MPO expression, pulmonary edema, and ultimately reversed lung cell apoptosis.
Due to its excellent antioxidant and anti-inflammatory properties as well as its ability to treat ALI, NPT@NP has great potential in the treatment of multiple lung diseases such as ARDS, asthma and pulmonary fibrosis.
Corresponding author: Changyou Gao
Authors: Wali Muhammad, Min Liang, Beiduo Wang, Wajiha Ahmed
Literature source: https://doi.org/10.1021/acs.biomac.4c01290
About GSHWORLD



