Research introduction abstract
The IDEALE study is an open, multicenter Italian study of the efficacy and safety of citicoline in older people patients with mild vascular cognitive impairment (VCI).
The study explored the clinical value of citicoline by assessing changes in cognitive function and quality of life, as well as the occurrence of adverse events, with the objective of evaluating the efficacy and safety of oral citicoline in the older people with mild vascular cognitive impairment.
Part 1 citicoline Research background
Vascular cognitive impairment (VCI) is a decline in cognitive function caused by cerebrovascular disease,
which is common in the older people population.
The clinical manifestations of VCI include memory loss and executive dysfunction, which seriously affect the quality of life of patients.
With the deepening of medical research, the treatment of VCI has gradually become a research hotspot.
Citicoline, as a neuroprotective agent,
has been studied worldwide for the treatment of ischemic and hemorrhagic stroke with a good safety profile.
The therapeutic effect of citicoline
1) Neuroprotective effect under hypoxia and ischemia;
2) Improved attention, learning and memory performance during brain aging in animal models;
3) Restore mitochondrial ATPase and membrane Na+/K+ ATPase activities;
4) Inhibited the activation of phospholipase A2 and accelerated the reabsorption of brain edema in various experimental models.
The IDEALE study builds on these neuroprotective effects to evaluate the efficacy of citicoline in older people patients with mild VCI.
Part 2 citicoline Materials and methods
The IDEALE study was an open, multicenter, randomized controlled trial conducted in six regions of Italy.
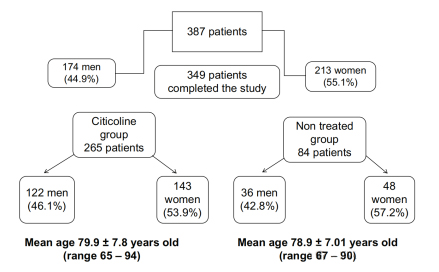
A total of 349 patients were enrolled in the study,
265 of whom received citicoline (the active group) and the rest were controls.
The patient was over 65 years of age,
had mild cognitive impairment as confirmed by the MMSE score, and neuroimaging showed vascular lesions.
The study was conducted in a double-blind, randomized,
placebo-controlled design to evaluate the efficacy and safety of citicoline in the treatment of mild VCI.
During the study, patients received either citicoline or a placebo for several months.
After treatment, the patients’ cognitive function changes were assessed by MMSE,
ADAS-Cog (Alzheimer’s Disease Assessment Scale-Cognitive part) and other scales, and the patients’ quality of life was assessed by quality of life scale.
Adverse events during treatment were recorded and analyzed.
Part 3 citicoline Research results
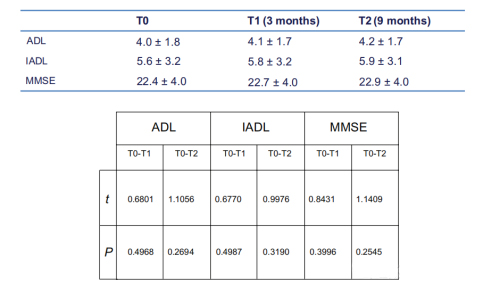
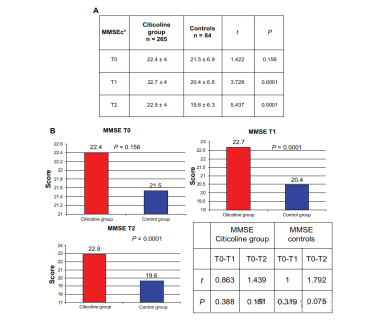
In the treatment group, the MMSE score remained largely unchanged over time
(22.4±4 at T0; At T1, the value was 22.7±4; At T2 it was 22.9±4).
Over the nine-month period of the study, the average improvement was 0.5 points, but there were no significant regional differences.
MMSE scores decreased at 9 months in the untreated group
(21.5 at T0; At T1, it was 20.4; At T2, it was 19.6; Decreased by 1.9 points between T0 and T2).
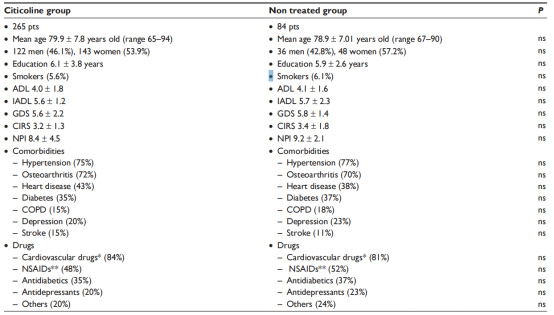
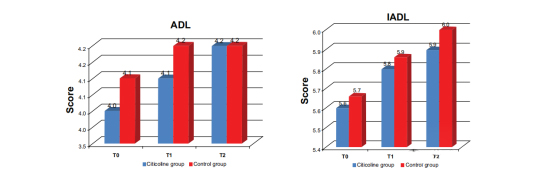
Activities of daily living (ADL) and instrumental Activities of daily living (IADL) scores did not differ between the two groups.
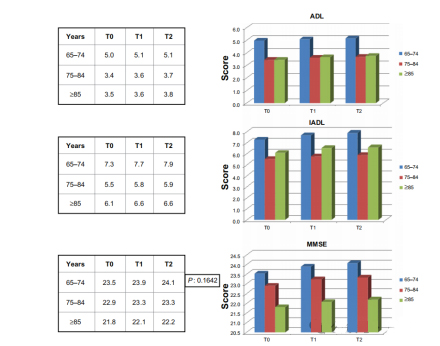
The study also explored possible differences in ADL, IADL, and MMSE scores among different age groups
(65-74 years in the younger group, 75-84 years in the older group, and ≥85 years in the older group).
Younger and older patients did better, but there was no significant difference compared to other age groups. There were significant differences in MMSE scores at T1 (P<0.0001) and T2 (P<0.0001) time points between the treatment and control groups, but no significant differences between T0 and T1 or between T0 and T2.
Overall, the DEALE results showed significant improvements in cognitive function and quality of life in patients treated with citicoline.
The improvement in MMSE and ADAS-Cog scores more significant in the active group compared with the control group. The active group also scored significantly higher on the quality of life scale than the control group.
In terms of safety, the incidence of adverse events was lower in the citicoline treatment group, and most of the adverse events were mild.
Part 4 Research and discussion
The results of the IDEALE study indicate that citicoline has a significant efficacy and good safety profile in the treatment of older people patients with mild VCI. The treatment group saw an improvement in the mini-mental State Examination (MMSE) score, showing an increase of 0.5 points over the study period.
The good results in clinical studies may be due to the fact that citicoline can slow the progression of ischemic cell damage by inhibiting the release of free fatty acids.
Some studies have also shown that citicoline appears to be a drug capable of providing “safe” neuroprotection by enhancing protective endogenous pathways.
These properties suggest that citicoline can recommended as a neuroprotective agent in patients with vascular cognitive impairment, vascular dementia, or Alzheimer’s disease with significant cerebrovascular disease, improving cognitive function and quality of life in patients with a low incidence of adverse reactions.
The IDEALE study still has some limitations.
The sample size of the study is relatively small, which may lead to some bias in the results. Second, the study did not stratify patients with different types and degrees of VCI, so it is not possible to determine the therapeutic effect of citicoline in different subgroups.
Future studies could further expand the sample size and conduct subgroup analyses to verify the clinical value of citicoline in the treatment of VCI.
Part 5 Research conclusion
The IDEALE study provides strong evidence for the efficacy and safety of citicoline in the treatment of older people patients with mild VCI.
Citicoline can significantly improve cognitive function and quality of life in patients with high safety.
This finding provides a new option for clinical treatment of VCI. Future studies may further explore the therapeutic effect and mechanism of citicoline in patients with different types of VCI.
Future research on whether the combination of citicoline and cholinesterase inhibitors can help delay the progression of Alzheimer’s disease is also an important direction of clinical value.
Research review content
This article analyzes the article “IDEALE Study – Efficacy and Safety of Citicoline in the treatment of mild vascular cognitive Impairment”, an important open, multicenter clinical trial to evaluate the efficacy and safety of citicoline in the treatment of older people patients with mild vascular cognitive impairment (VCI).
The results of this study indicate that citicoline can significantly improve the cognitive function and quality of life of patients with good safety, providing a new option for clinical treatment of VCI.
This article has a few highlights:
(1/ Significant effectiveness: Compared with the control group, patients treated with citicoline showed significant improvements in both MMSE and ADAS-Cog scores, as well as significantly higher quality of life scores than the control group. This suggests that citicoline can effectively delay the cognitive decline and improve the quality of life in VCI patients.
(2/ Good safety: In the study, the incidence of adverse events in the Citicoline treatment group was lower, and most of the adverse events were mild, indicating that Citicoline has a good safety and is suitable for long-term treatment of older people patients with VCI.
(3/ Clear mechanism of action: Citicoline, as a neuroprotective agent, can act through a variety of mechanisms, including inhibiting free fatty acid release and enhancing endogenous protective pathways, thereby delaying cognitive decline in patients with VCI. At the same time, the relatively small sample size and the lack of subgroup analysis are two disadvantages.
Sum up
This article provides strong evidence for the efficacy and safety of citicoline in the treatment of VCI. Citicoline, as a safe and effective neuroprotective agent, expected to a new choice for the treatment of VCI. Future studies can further explore its mechanism of action and combination therapy to bring more benefits to patients with VCI.
Literature source:
[1]Antonino Maria,Cotroneo,Alberto,Castagna,Salvatore,Putignano,Roberto,Lacava,Fausto,Fantò,Francesco,Monteleone,Filomena,Rocca,Alba,Malara,Pietro,Gareri.Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study.[J].Clinical interventions in aging,2013,8:131-7.