What is L-tyrosine
L-tyrosine is an aromatic non essential amino acid that is a raw material for many important chemical products, including levodopa, resveratrol, and hydroxytyrosol.
Widely used in industries such as food, feed, chemicals, and pharmaceuticals. It is a precursor to various natural products, including caffeic acid, flavonoids, curcumin, 3,4-dihydroxy-L-phenylalanine (L-DOPA), p-hydroxystyrene, and p-hydroxycinnamic acid.
The demand for L-tyrosine in the market continues to rise, with market prices reaching around 800000 yuan per ton. The traditional production methods of T include protein hydrolysis, chemical synthesis, enzymatic method, and microbial fermentation. Protein hydrolysis and chemical synthesis methods have been gradually phased out, and currently the main production methods are enzymatic and microbial fermentation.
Production methods
Enzymatic method based on β- The reaction in which tyrosinase converts substrates such as phenol L-serine or phenol pyruvate ammonia into L-tyrosine. The disadvantage high cost and poor stability.
In addition, the phenol used in the production process is highly toxic, corrosive, and extremely harmful to the environment.
Microbial fermentation uses simple carbon sources as raw materials, and its advantages include low cost, simple process, and low environmental burden. Microbial de novo synthesis of L-tyrosine is considered to have very broad development prospects.
Despite extensive research on the shikimic acid pathway over the past 40 years, utilizing microorganisms to synthesize L-tyrosine still faces many challenges, such as low metabolic flux of the shikimic acid pathway and complex metabolic regulation involved in gene manipulation. In the feed batch fermentation process, strains require long-term high-density and high-intensity production.
Currently, with the development of metabolic engineering and biotechnology, it has become easier and more feasible to design microbial metabolic pathways to achieve high accumulation of L-tyrosine.
To achieve efficient production of L-tyrosine in Escherichia coli, Professor Zhou Jingwen’s team and Professor Zhou Zhemin’s team modified the Tyr synthesis pathway to obtain strains with efficient production products.
This achievement published in “Synthetic and Systems Biotechnology”,Synergetic engineering of Escherichia coli for efficient production of L-tyrosine,https://doi.org/10.1016/j.synbio.2023.10.005
In this study, in order to increase the production of L-tyrosine in Escherichia coli, the expression of key enzymes in the shikimic acid pathway upregulated or downregulated. Modify the Tyr transport system and acetic acid biosynthesis pathway to further enhance the production of L-tyrosine.
In addition, a combination of phosphoketolase pathway and cofactor engineering introduced to redirect carbon flux to the shikimate pathway. Finally, after evolving to low pH values in the adaptive laboratory, the optimal strain obtained. The strain produced 92.5g/L L-tyrosine in a 5L fermentation tank, with a yield of 0.266g/g glucose.
Firstly, by overexpressing or knocking out genes to alter metabolic pathways in cells, high-yield strains can be screened.
Select Escherichia coli WSH-Z06 as the starting strain. Several genes related to the accumulation of shikimic acid and aromatic amino acids, such as aroG, tyrA, tktA, ppsA, aroA, aroB, aroC, aroE, aroL, tyrR, pheA, trpE, adhE, pflB, ldhA, were screened.
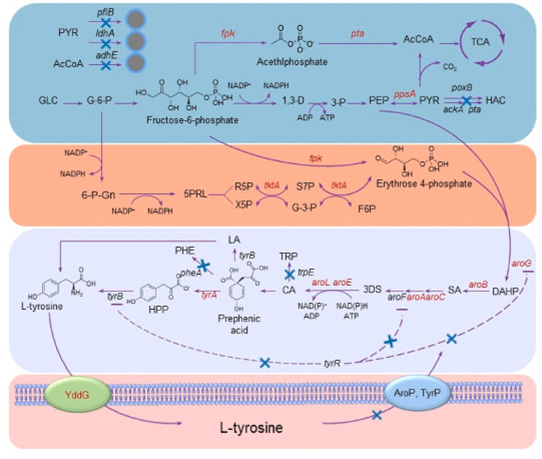
Knockout or overexpression of these genes can have an impact on the accumulation of L-tyrosine, with the combined knockout of tyrR, pheA, and trpE resulting in the highest production of L-tyrosine (3.76g/L).
Knocking out tyrR can alleviate the inhibition of shikimate pathway enzymes, and on this basis, increase the supply of phosphoenolpyruvate and erythritol 4-phosphate precursors in the shikimate pathway, block the branching metabolism of tryptophan and phenylalanine synthesis, and ultimately accumulate 4.22g/L L-tyrosine in the applied strain within 48 hours.
Secondly, improve the L-tyrosine transport system.
Knockout the AroP and TyrP genes of the aromatic amino acid transporter and express the YddG aromatic amino acid export protein, obtaining an engineered strain that increases L-tyrosine efflux and reduces intracellular L-tyrosine concentration.
Thirdly, the introduction of PK pathways combined with cofactor cycling.
In order to further promote the accumulation of L-tyrosine, the PK pathway introduced, directing glucose towards the shikimic acid pathway.
Connect endogenous genes ppsA, tktA, and yddG with constitutive strong promoters and integrate them into the strain genome.
When expressing phosphoketolase (fpk) and endogenous phosphoacetyltransferase (pta) in Escherichia coli, the accumulation of L-tyrosine significantly reduced.
After adding the cofactor NADH, the growth of the strain resumed and the accumulation of Tyr increased. Linking the endogenous udhA and pntAB genes of Escherichia coli with strong promoters increases the mutual transformation between NADPH and NADH in cells, and the strain can accumulate 6.17/L of L-tyrosine within 48 hours.
The modification of glycolysis and shikimic acid pathway enhances the flux of shikimic acid pathway.
However, with the introduction of FPK, some carbon metabolic flows directly transferred to the shikimic acid pathway through the PK metabolic pathway, further reducing the flux through the glycolytic pathway.
Given that the glycolytic pathway the main source of NADH production, this change,
combined with previous modifications, leads to an imbalance of cofactors in Escherichia coli,
which may be the reason for the decrease in L-tyrosine production.
Therefore, adding NADH during the induction process can significantly increase the accumulation of L-tyrosine.
Finally, changing the acetic acid pathway to shorten fermentation time.
When the strain fermented in batches in a 5L fermentation tank for 69 hours,
it could accumulate 50.2g/L of L-tyrosine, but 12.4g/L of acetic acid detected in the fermentation broth.
Acetates in Escherichia coli mainly produced by the AckA PTA pathway and pyruvate oxidation pathway,
but knocking out genes in the pathway can affect the growth of Escherichia coli.
Therefore, it is necessary to further improve the tolerance of Escherichia coli to acetic acid and carry out acid adaptive evolution.
More than 200 strains were screened for 50 passages,
and 24 strains that grew well at pH 5.1 were ultimately screened to verify the production of Tyr.
By controlling the fermentation conditions such as temperature and pH,
initial sugar concentration, and dissolved oxygen in a 5L fermentation tank,
the fermentation performance of the experimental strains can improved, while using a reflux fermentation method.
By adjusting the feeding rate of fermentation, the yield of L-tyrosine reached 92.8g/L.
Although the glucose conversion rate decreased by 4.3%, the yield of L-tyrosine increased by 12.3g/L,
and the fermentation time shortened by 10 hours, which of great significance for the industrial production of Tyr.
Global Biosynthesis Status of L-Tyrosine
The biosynthetic pathway of L-tyrosine

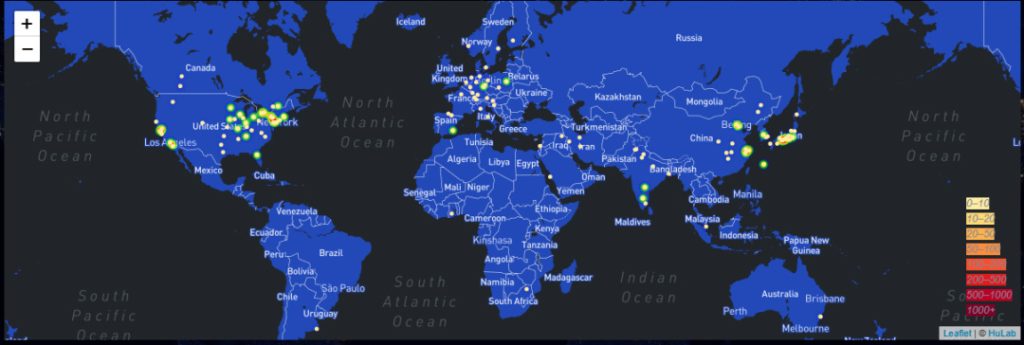
Global L-Tyrosine Technology Power Distribution Map