Rutgers University, USA: NMN reduces heart damage after myocardial infarction
The coronary artery is the main blood vessel that supplies blood to the heart. It starts in the large aorta at the top of the heart and gradually branches downward.
Under the influence of aging, high blood pressure, high blood lipids and other factors, coronary arteries will become blocked and lead to myocardial cell ischemia, at which time patients will have chest pain, breathing difficulties and other symptoms, which is commonly known as heart attack.
Without timely treatment, the ischemic heart muscle cells will gradually die and the patient will die from heart failure.
The primary goal of clinical treatment of myocardial infarction is to restore blood supply as soon as possible. However, since cardiomyocytes gradually appear a series of “abnormal states” from the beginning of ischemia, they often cannot quickly adapt to the original “normal state” when they come into contact with fresh blood again, which will lead to further damage to the heart. This is called ischemia/reperfusion (I/R) injury.
In 2014, researchers at Rutgers University New Jersey Medical School found that Ischemic preconditioning (IPC) training can reduce reperfusion damage after an actual heart attack in mice. What’s more, injecting mice with NMN (niacinamide mononucleotide) can “mimic” this “training” and achieve the same effect.
The study was published in the journal PLOS One in June of the same year.
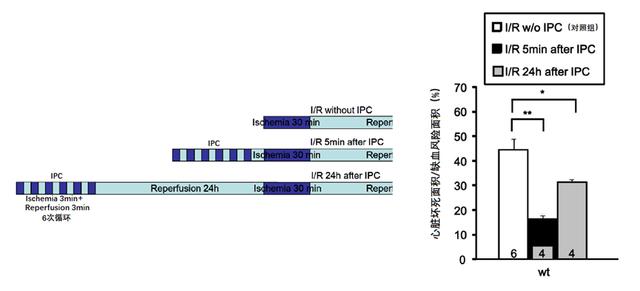
In the experiment, the researchers performed open-heart surgery on mice and lapped some blood vessels in their coronary arteries using sutures and silicone tubes, at which time an electrocardiogram showed that the mice’s hearts were already suffering from ischemia.
After some time, the researchers removed the sutures and silicone tubes, allowing the mice’s hearts to be reperfused with blood. The results showed that, compared with the control group, the mice that underwent a short period of repeated ischemia-reperfusion “training” had a smaller area of myocardial necrosis after the actual 20 minutes of cardiac ischemia and reperfusion.
The effect was most pronounced in the first five minutes after the “training”, but also maintained 24 hours later.
Further studies showed that ischemic preconditioning activated increased expression of an enzyme called Nampt in mice.
Nampt is an enzyme that catalyzes NAM (nicotinamide) to synthesize NMN in vivo, and NMN rapidly converted into NAD+ by organisms and exerts a series of biological effects.
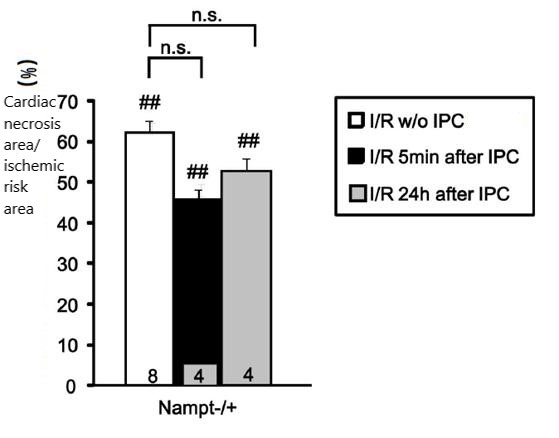
The researchers performed the same ischemia/reperfusion experiment on gene-edited mice with low Nampt expression (Nampt-/+ mice) and found that not only the area of cardiac necrosis significantly increased after reperfusion, but the cardiac protective effect of ischemic preconditioning also lost.
Since Nampt plays a role in the synthesis of NMN in vivo,
increased expression of Nampt often means increased levels of NMN,
so the researchers hypothesized that direct supplementation of NMN may be equally effective.
In the following ischemia-reperfusion surgery,
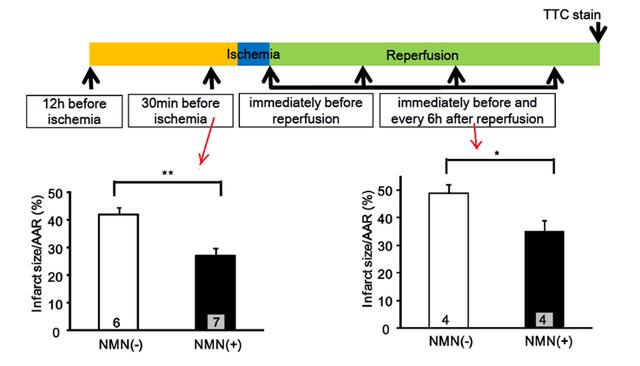
the researchers selected different times to inject 500mg/kg (equivalent to 2.25g in humans) of NMN into the mice,
the specific injection process shown in the following figure.
The results showed that continuous injection of NMN 30 minutes before and after ischemia significantly reduced ischemia/reperfusion injury in mouse hearts.
In summary, this study found that the “training” of the mouse heart through ischemic preconditioning can effectively reduce the ischemia/reperfusion injury after myocardial infarction;
Injection of NMN can “simulate” the process of ischemic preconditioning and achieve the same cardiac protection effect. Considering that it is not possible to “train” patients with coronary artery disease to undergo ischemic preconditioning in actual treatment,
NMN therapy clearly has greater clinical potential.
In addition, in 2020, researchers at the University of Maryland in the United States found that NMN treatment can also reduce ischemic brain injury after cerebral infarction in mice,
1 which further verified the key role of NMN in combating post-ischemic injury.
reference
- 1. Klimova, N., Fearnow, A., Long, A., & Kristian, T. (2020). NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Experimental neurology, 325, 113144.
- 2. Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., & Sadoshima, J. (2014). Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9(6), e98972.